Abstract
Infection with the gram-negative bacterium Burkholderia pseudomallei can result in a life-threatening disease known as melioidosis. Historically, melioidosis was a common infection in military forces serving in Southeast Asia, and it has the potential to have a serious impact on force health readiness. With the U.S. Department of Defense (DOD)'s increasing strategic and operational focus across the Pacific Theater, melioidosis is an increasingly important issue from a force health protection perspective. U.S. Marines deploy annually to Darwin, Australia, a "hyperendemic" region for B. pseudomallei, to engage in training exercises. In an effort to assess the risk of B. pseudomallei infection to service personnel in Australia, 341 paired samples, representing pre- and post-deployment samples of Marines who trained in Australia, were analyzed for antibodies against B. pseudomallei antigens. Serological evidence of possible deployment-related infection with B. pseudomallei was found in 13 Marines. Future prospective studies are required to further characterize the risk to service members deployed to melioidosis endemic areas.
What Are the New Findings?
Since 2012, U.S. Marines have participated in training exercises in Darwin, Australia, one of the world's "hyperendemic" regions for Burkholderia pseudomallei. Analysis of pre- and post-deployment serum samples obtained from the Department of Defense Serum Repository identified serological evidence of possible infection with B. pseudomallei in U.S. Marines who trained in Australia during 2012–2014.
What Is the Impact on Readiness and Force Health Protection?
Historically, melioidosis was a common infection in military forces serving in Southeast Asia, and it has the potential to have a serious impact on force health readiness today. Given the U.S. Department of Defense's increasing strategic and operational focus across the Asia-Pacific Theater, melioidosis is an increasingly important issue from a force health protection perspective.
Background
Melioidosis is a potentially life threatening disease caused by the gram-negative bacterium Burkholderia pseudomallei. Endemic to tropical regions, melioidosis is especially prevalent in Southeast Asia (Thailand, Malaysia, Singapore, Cambodia, Vietnam, and Myanmar) and northern Australia.1,2 A recent model estimated an incidence of 165,000 melioidosis cases per year (an incidence rate of 5.0 per 100,000 people at risk), with a predicted mortality of 89,000 per year, among the 3 billion people residing in areas likely to contain B. pseudomallei.3 The true global distribution of B. pseudomallei and the incidence of melioidosis remain poorly understood, and it is not yet known if the growing number of melioidosis cases reported worldwide reflects an unmasking of long-standing bacterial presence or the spread of B. pseudomallei to previously unaffected areas.4
The primary route of infection with B. pseudomallei is believed to be through skin inoculation of the soil-dwelling bacterium. Inhalation, aspiration, or ingestion from contaminated water sources is also common.4,5 The bacterium can infect any organ in the body, precipitating a diverse assortment of clinical presentations. Patients may experience asymptomatic seroconversion; a mild illness manifesting as non-specific febrile symptoms; or a severe, systemic infection resulting in abscess formation, pneumonia, and fatal septic shock.6 Latent infection with subsequent activation can also occur but has been very uncommon according to data from the Darwin Prospective Melioidosis Study.7
The indirect hemagglutination assay (IHA) is the most widely used serological test for the detection of antibodies directed against B. pseudomallei.8–10 While the clinical utility of the IHA in both melioidosis-endemic and non-endemic settings is well described, persistently negative IHA findings in culture-confirmed cases of melioidosis, decreasing titers in serial samples, and high background seropositivity in endemic locations are all well documented.11–13 In addition, performing the IHA is cumbersome, making it impractical for screening large numbers of samples. The utility of the IHA is further limited by the fact that its results may vary depending on the population being tested and that the reagents are difficult to obtain in non-endemic areas. Expert commentary has acknowledged the need to develop and validate alternative serological tests, especially for pre- vs post-exposure surveillance.10
Enzyme-linked immunosorbent assays (ELISAs), when antigens are carefully selected and properly validated, represent a potentially superior method for detecting B. pseudomallei infection, especially when pre- and post-exposure samples can be examined.10,14,15 Two antigens from B. pseudomallei are undergoing clinical validation in an effort to provide accurate, inexpensive, and reproducible assays for the detection of B. pseudomallei infection. An O-polysaccharide (OPS)-based ELISA (OPS-ELISA) was recently evaluated using serum samples from culture-confirmed melioidosis patients from Thailand, patients with other bacterial infections, and healthy donors from northeast Thailand and the U.S.16 The OPS-ELISA displayed a sensitivity of 71.6% among culture-confirmed patients, a specificity of 95.7% for healthy Thai controls, and a specificity of 96.7% for healthy U.S. controls, demonstrating the potential for a superior serological assay for melioidosis.16 Hemolysin co-regulated protein 1 (Hcp1) is another promising target for serological assays.17,18 While the Hcp1-ELISA was more sensitive than the OPS-ELISA (83.0% versus 71.6%), both had specificity greater than 95%.18 Although more studies are needed to determine how broadly these results can be applied, Hcp1- and OPS-based assays represent viable platforms for the detection of antibodies directed against B. pseudomallei, especially in an immunologically naïve population.
Historically, melioidosis has been a serious threat to foreign military forces deployed to Southeast Asia. Foreign troops are often immunologically naïve and are potentially exposed to environmental B. pseudomallei percutaneously through skin abrasions, burns, and combat wounds or by inhalation through aerosolization of B. pseudomallei from blasts, helicopter rotor blade updraft, or severe weather events.6 For instance, numerous cases have been reported in helicopter crews, likely due to inhalation of dust and aerosolized water during dustoffs.19,20 Between 1948 and 1954, at least 100 French troops developed melioidosis while serving in Indochina.19 During the Vietnam conflict, 343 cases of melioidosis were reported in U.S. soldiers by 1973. Furthermore, an estimated 225,000 U.S. military personnel returning from Vietnam exhibited serological evidence of infection.21 Termed "the Vietnamese time bomb," reactivation from latent foci was well documented among veterans of the Vietnam War years after return to the U.S.22–27
Today, melioidosis remains a force health protection challenge to the U.S. military. For example, in May 2006, a previously healthy U.S. Marine developed severe systemic melioidosis following a 2-week military exercise in Thailand.28 He was treated with an intensive 12-month course of multi-antibiotic therapy and experienced full recovery. As the U.S. Department of Defense (DOD) continues to increase its focus toward the Asia-Pacific Theater, melioidosis has the potential to threaten U.S. military members serving in B. pseudomallei-endemic areas.
In April 2012, U.S. Marines began deploying to Darwin, Australia, to participate in joint training exercises with the Australian Defense Force. Marine Rotational Force–Darwin (MRF-D) began with a modest force of approximately 200 Marines and has grown to over 1,500 Marines. It is expected to eventually grow to over 2,500 Marines and sailors. Current MRF-D rotations last approximately 6 months and are timed to avoid the wet season, when the majority of melioidosis cases occur. However, the duration of rotations may increase in the future, and there have been several cases of melioidosis in Australian military personnel undertaking training in the same location in northern Australia over the last 2 decades7 (also unpublished data, Dr. Bart J. Currie, Royal Darwin Hospital), indicating that U.S. Marines and sailors may be at risk. But, no melioidosis serosurveys have been undertaken by the Australian military, so the seroprevalence is unknown.
This study sought to understand the risks of B. pseudomallei infection in U.S. Marines serving in Australia by first characterizing the rates of infection in archived serum samples. This report describes serological evidence of possible asymptomatic B. pseudomallei infections in U.S. Marines serving in endemic areas.
Methods
Serum samples
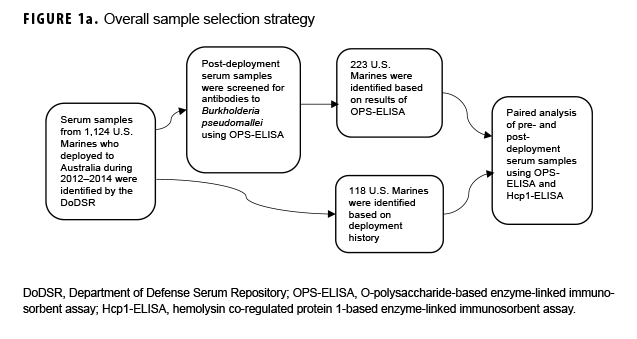
De-identified serum samples were obtained from the DOD Serum Repository (DODSR).29 Samples from 1,124 U.S. Marines with deployment histories that included deployment to Australia between 2012 and 2014 were located in the DODSR by Armed Forces Health Surveillance Branch staff. Information on demographic and military characteristics, serum collection dates, sex, military occupational specialty, and deployment history was provided. The exact locations of deployment sites (e.g., Darwin) and training areas in Australia were not captured.
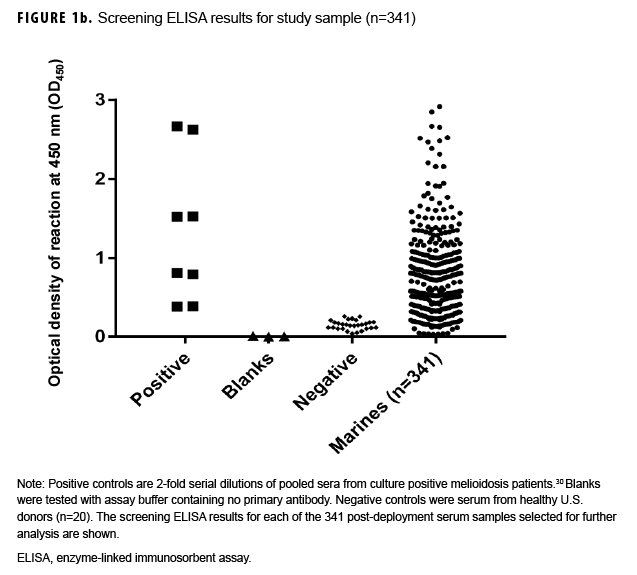
Paired sample selection (Figure 1a) began by screening post-deployment samples for antibodies to the B. pseudomallei type A OPS (Figure 1b) using a 1:2,000 dilution as previously described.16 In the absence of known cutoff values for U.S. samples, a total of 223 individuals representative of the screen results (i.e., highly positive through negative; Figure 1b) were selected for further testing. To address potential selection bias associated with the screening results, an additional 118 unscreened individuals whose only deployment was to Australia in 2014 were identified. Pre-and post-deployment samples from each of these 341 individuals were paired and analyzed as described below.
Serological analyses
Antibody capture ELISAs targeting the B. pseudomallei Hcp1 and type A OPS antigens were performed as previously described, with minor modifications to the sample preparation.16 Briefly, 3-fold serial dilutions of the serum were used to generate curves ranging from 1:74 to 1:54,000. Paired titers were run in duplicate and positive results were verified by repeated analysis. Negative controls were commercially obtained serum samples from U.S. sources (Biological Specialty Corporation, Reading, PA 19602), while positive control sera were derived from culture-confirmed melioidosis cases from Cambodia.30 Pre-deployment and post-deployment sera for each individual were paired for analysis. IHAs were performed at the Royal Darwin Hospital as previously described.8,9,12
Statistical analysis
Paired pre- and post-deployment sera were evaluated with a paired 2-tailed t test using GraphPad Prism version 6.0 (2013, GraphPad Software, La Jolla, CA) and Stata version 14 (2015, StataCorp LP, College Station, TX). Statistical significance was defined as p<.05.
Results
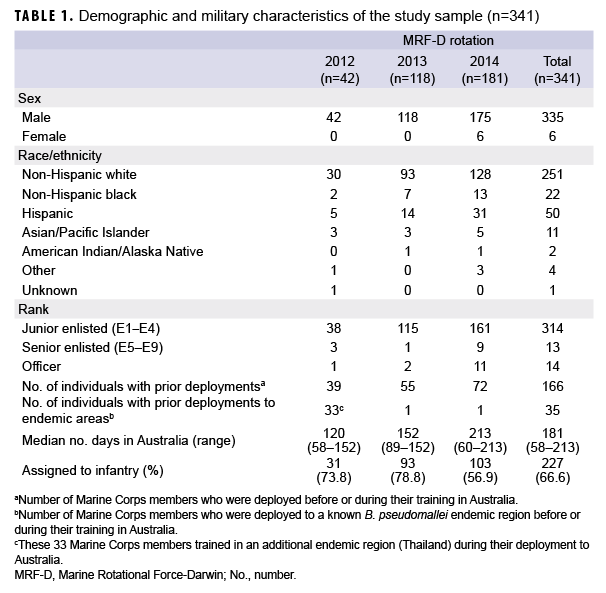
Demographic and military characteristics of the study sample (n=341) are presented in Table 1. The individuals were heavily skewed toward white and male (251/341 and 335/341, respectively) junior enlisted Marines (314/341). Australia was the first deployment for over half (51.3%) of the study sample. Of those with prior deployments, 131/166 (78.9%) were deployed to non-endemic areas (e.g., Afghanistan, Iraq, Japan). During the 2012 rotation, it was common practice for Marines to also travel to Thailand for a short period of training (2–4 weeks); more than three-quarters (33/42) of those with an MRF-D rotation in 2012 had travel histories that included Thailand (Table 1).
Serological evidence of infection with B. pseudomallei using the OPS-ELISA
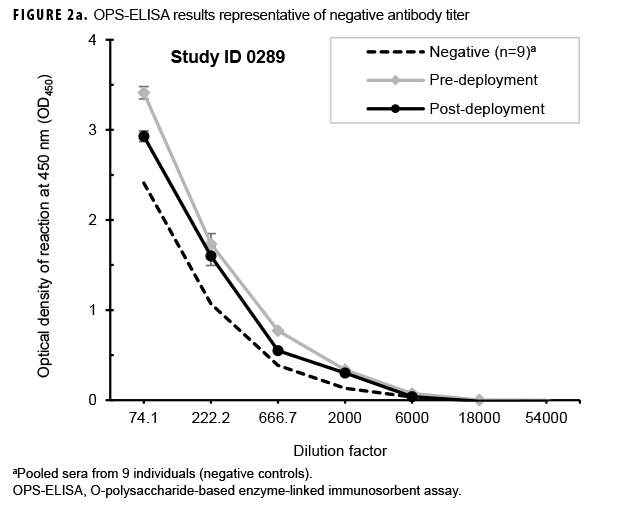
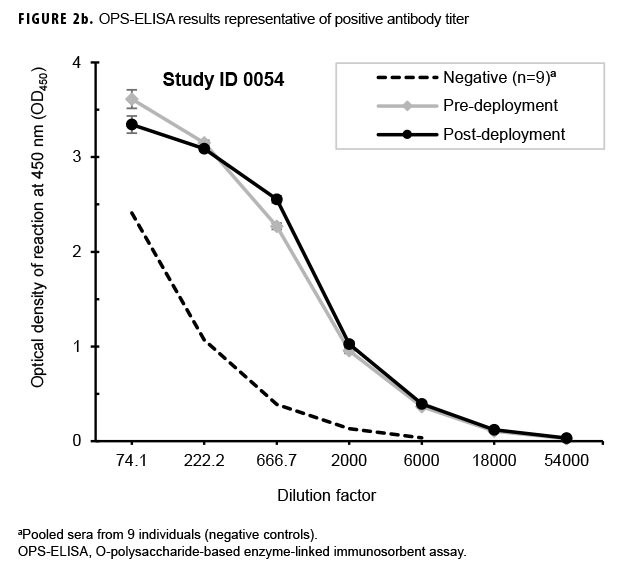
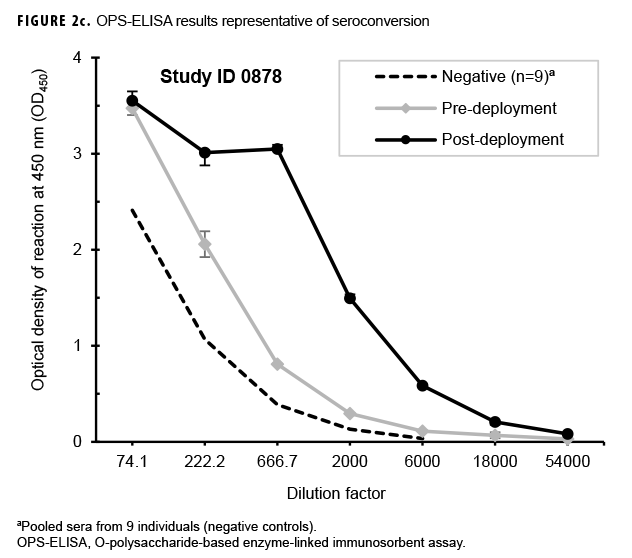
Paired sera samples analyzed for antibodies against the OPS-ELISA produced 3 patterns of results (Figures 2a, 2b, 2c). The vast majority (78.9%; 269/341) of the Marines in the study lacked detectable OPS antibodies in both their pre- and post-deployment sera samples (Figure 2a). Fifty-nine Marines displayed elevated antibody titers in both their pre- and post-deployment samples (Figure 2b). Finally, 13 Marines appeared to have seroconverted to B. pseudomallei OPS during their deployment to Australia, as their post-deployment serum samples displayed statistically significant increases in antibody titer (Figure 2c).
Hcp1-ELISA–based evidence of infection with B. pseudomallei
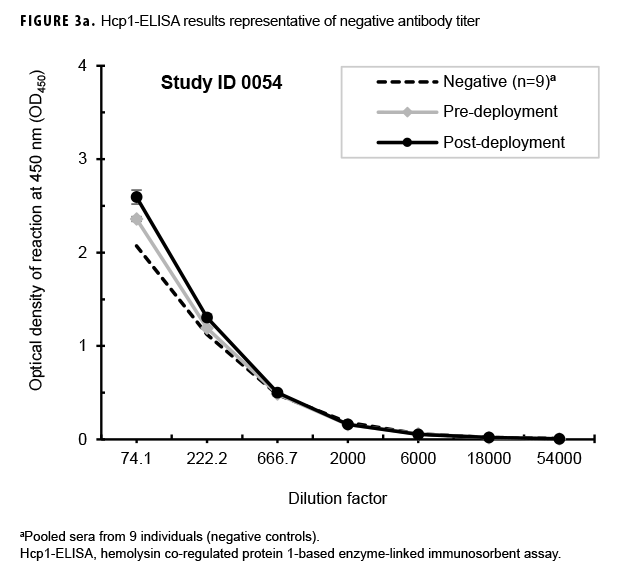
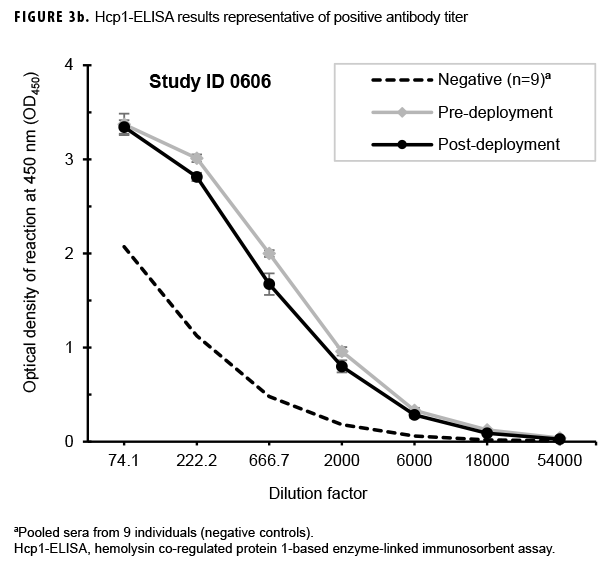
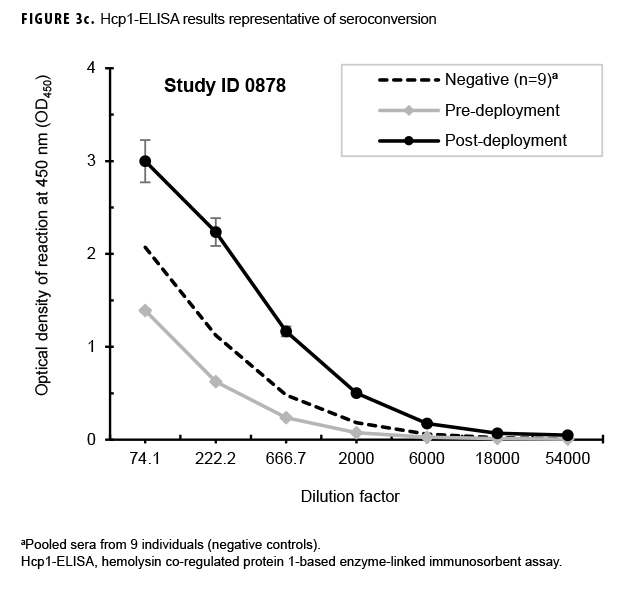
Paired sera samples analyzed for antibodies against Hcp1 were broadly consistent with OPS-ELISA results and yielded similar categories of results (Figures 3a, 3b, 3c), although with interesting differences. The number of pairs with detectable antibodies in both their pre- and post-deployment samples fell by 51 (compare Figure 2b to Figure 3a), resulting in only 8 Marines with elevated anti-Hcp1 pre- and post-deployment titers (Figure 3b). Using Hcp1-ELISA, paired sera from 12 Marines, including 10 who were OPS-ELISA positive, displayed statistically significant increases in their post-deployment samples relative to their pre-deployment samples (Figure 3c). While 3 individuals demonstrating seroconversion to OPS showed no detectable antibodies to Hcp1, the Hcp1-ELISAs resulted in the identification of 2 additional seroconverters not seen with the OPS-ELISA.
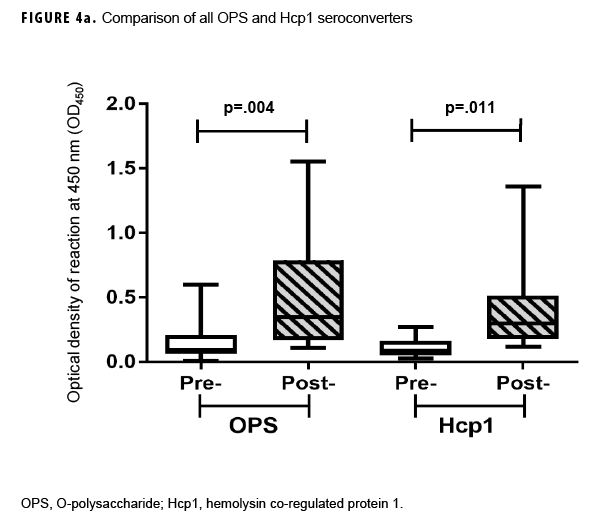
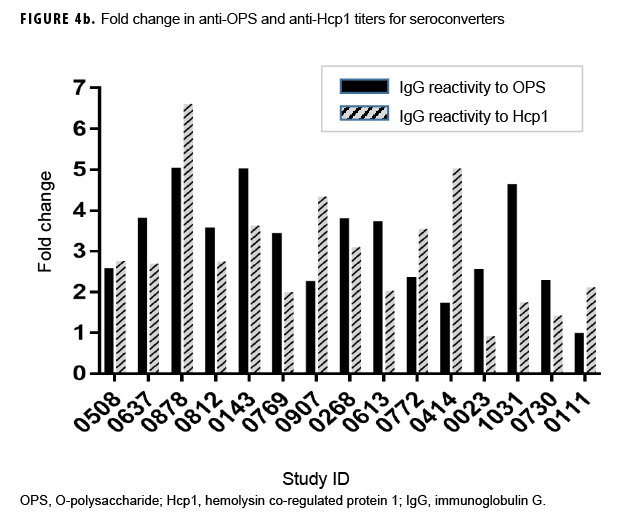
Previous studies employing the OPS- and Hcp1-ELISA have found that the 1:2,000 dilution of serum produces highly consistent and discriminating (i.e., melioidosis vs non-melioidosis) results.2,16,18 Here, a paired 2-tailed t test was used to analyze the results from each pair of samples that exhibited seroconversion across the entire dilution series. These analyses determined that the 1:2,000 dilution provided the most consistent resolution (p=.004 and .0105 for OPS and Hcp1, respectively) for these samples as well (Figures 2c, 3c). The optical density (OD)450 values at 1:2,000 for these individuals were then compared by a paired 2-tailed t test and found to be significantly different for each antigen (Figure 4a). Finally, the OD450 values of paired pre- and post-deployment sera at 1:2,000 were used to calculate fold change in anti-OPS and anti-Hcp1 titers (Figure 4b).
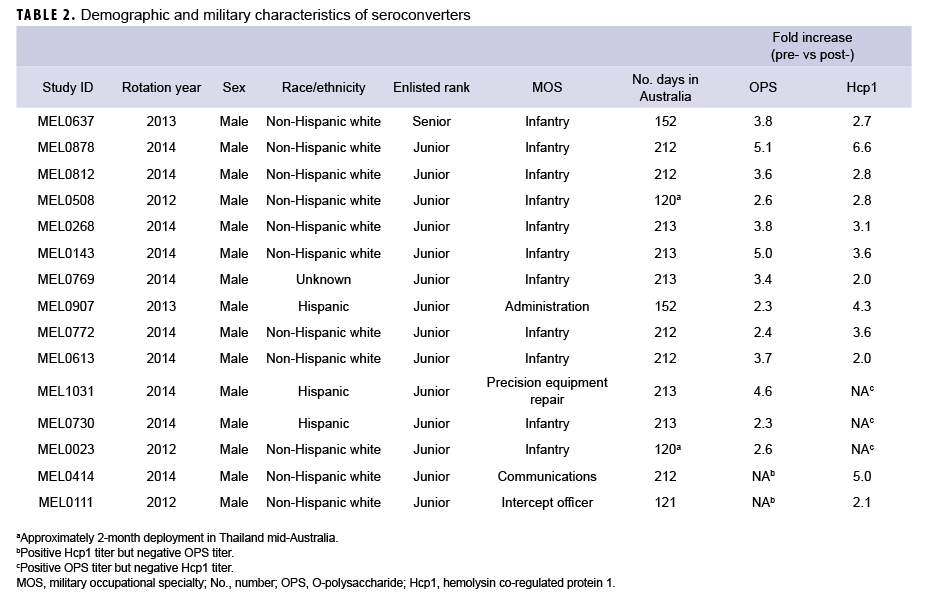
In summary, 15 Marines demonstrated seroconversion to B. pseudomallei antigens; 13 of these Marines showed at least a 2-fold increase in OPS titers, and 12 had increased Hcp1 titers (Table 2). A greater than 4-fold increase in antibody titer occurred in 3 of the 13 for OPS-ELISA and 3 of the 12 for Hcp1-ELISA (Figure 4b). Ten of these Marines overlapped in the 2 groups, 3 Marines had increasing OPS titers but no Hcp1 titer, and 2 Marines who were OPS positive in both pre- and post-deployment samples demonstrated deployment-related seroconversion to Hcp1.
IHA serology results
To begin to understand the relationship between IHA and OPS/Hcp1-ELISAs in a U.S. population, samples from 22 individuals were selected to be analyzed by IHA as previously described.8,31 These samples included pre- and post-deployment sera from 9 Marines who seroconverted to OPS (Figure 2c), 5 Marines who were positive for OPS antibodies in both pre- and post-deployment sera (Figure 2b), and 8 who were negative in both samples (Figure 2a). All IHA titers registered at either <1:20 or 1:20, titers considered negative in Darwin, Australia.12 However, 4 individuals who seroconverted to OPS and/or Hcp1 were <1:20 in their pre-deployment sample and 1:20 in their post-deployment sample, potentially indicating low-level seroconversion by IHA.
Editorial Comments
Melioidosis among deployed military forces is well described,9–21,27,28,32–34 and it is an emerging public health problem in tropical regions, with increases in incidence reported in multiple regions of Southeast Asia3 and in northern Australia.35 Even in non-resource–limited settings, the reported mortality of acutely diagnosed melioidosis is high and ranges between 9–18% in recent reports from Australia and Singapore.36,37 The diagnosis of melioidosis can often be delayed given its heterogeneous presenting syndrome and the fact that empiric sepsis therapy in non-endemic regions often excludes the antimicrobial agents used in melioidosis treatment (i.e., ceftazidime, imipenem, and meropenem).38 With the DOD's increasing strategic and operational focus throughout melioidosis-endemic areas, this disease is important for force health protection, and deployment history should be considered by treating physicians.
OPS is a major structural component of lipopolysaccharide and has been shown to be a dominant antigen recognized by antibodies in melioidosis patient sera.15,39,40 Three serologically distinct OPS types (A, B, and B2) have been described for B. pseudomallei strains.41,42 Of these, type A OPS is the predominant antigen expressed by the majority of strains described to date. Interestingly, the proportion of strains expressing the different OPS types can vary by region. For example, in Thailand, 97.7% of B. pseudomallei isolates express type A OPS, and in Australia, 85.3% express type A OPS, with 13.8% expressing type B OPS.42 Studies characterizing the structures of these OPS antigens show that they appear to be unique to Burkholderia species,43,44 a feature that is beneficial for the development of serodiagnostic assays. It should be noted, however, that some non-pathogenic, environmental near-neighbor species express OPS moieties that are the same or similar to B. pseudomallei OPS (e.g., B. thailandensis type A and B. humptydooensis type B2) and could potentially yield false-positive results.45 To help address this concern, additional target antigens (e.g., Hcp1) have been developed as serodiagnostic tools, and these have also been included in this study. More research is needed to determine whether or not serotype-specific OPS (A, B, or B2) assays may be useful for certain populations and environments under study.
Hcp1 expression is tightly regulated in B. pseudomallei. Several studies have shown that Hcp1 is highly expressed in vivo and following uptake by host cells but is undetectable when B. pseudomallei is cultured in vitro in rich media conditions.46,47 Hcp1 is immunogenic and stimulates high antibody titers in melioidosis patients.17 These features make Hcp1 a potentially attractive indicator of asymptomatic infection and clinical melioidosis because replication within the host is a requirement for anti-Hcp1 antibody production. Furthermore, because Hcp1 expressed by B. pseudomallei is structurally different from homologous proteins found in other Burkholderia species, an Hcp1-based assay may provide higher specificity by discriminating between exposure to B. pseudomallei and neighbor species (e.g., B. thailandensis).18 Finally, use of Hcp1 should also allow for the identification of individuals exposed to B. pseudomallei strains expressing type B OPS that would be missed using the type A OPS-specific ELISA described above.
More research is needed to determine if individuals who seroconverted to OPS but remained negative by Hcp1 may represent seroconversion to near neighbors or transient exposure to B. pseudomallei with rapid clearance of infection. Determining whether serology assays using Hcp1 or other specific B. pseudomallei antigens will help differentiate between transient and persisting but still asymptomatic infection with B. pseudomallei will require further prospective studies. Such an assay would be very useful to future efforts to characterize the risks to MRF-D.
Reconciling the low IHA results of the Marines when compared with the OPS-and Hcp1-ELISA results in this report is a critical issue for further study. IHA positivity on initial blood samples from patients presenting with acute melioidosis in Darwin has been as low as 56%.12 This reflects the rapidity of disease onset after infection, and the large majority of surviving initially seronegative patients with melioidosis seroconvert on IHA after hospital admission.48 However, up to 15% of culture-positive melioidosis patients may remain persistently negative by IHA.11,12 Additionally, longitudinal analysis demonstrated that approximately 8% of IHA-positive melioidosis patients lose their positive titers over time.12 Here, 4 Marines seroconverted to OPS and/or Hcp1 and demonstrated a weakly-increasing IHA titer (<1:20 pre-deployment to 1:20 postdeployment). It is unclear if these results truly represent infection with B. pseudomallei during deployment. Nine Marines who seroconverted by OPS and/or Hcp1 assays failed to produce any demonstrable reaction to the IHA. It is speculated that conflicting IHA and enzyme immunoassay results may represent low or decreasing IHA titers that have been identified by the more sensitive ELISA platform.49
Several cases of melioidosis have occurred in Australian military service members operating in the same training areas in northern Australia over the last 2 decades7 (also unpublished data, Dr. Bart Currie, Royal Darwin Hospital), but no melioidosis serosurveys have been undertaken in the Australian military. The current serological survey of a cohort of archived samples from U.S. Marines who trained in Australia found possible evidence of B. pseudomallei infection in the form of antibodies directed against the in vivo expressed B. pseudomallei protein Hcp1. A serosurvey of a healthy cohort of active civilians in the same region of Australia showed high IHA levels (titers =1/80) in 11/354 individuals, some of whom had a specific but transient clinical illness thought to possibly represent melioidosis that resolved without therapy.50
Limitations during this study did not allow for definitive conclusions to be drawn in several areas. Specifically, the metadata lacked detailed information about locations in Australia, clinical information regarding symptoms experienced during training, and background data such as birthplace and personal travel to explain background levels of antibodies directed against B. pseudomallei. Importantly, the study sample was not a random sampling of a rotation. A prospective study would provide more detailed subject data and a representative cross section of a rotation to more fully characterize the risks of melioidosis to MRF-D.
It is now recognized that melioidosis is predominantly a disease of those with predisposing risk factors and that mortality is strongly correlated with the presence of risk factors such as diabetes.4 Young, healthy adults, such as active duty U.S. Marines, lack these risk factors, and the vast majority of those infected with B. pseudomallei will have no clinical illness. What remains uncertain is how many asymptomatic people with positive serology, presumably reflecting infection with B. pseudomallei at some time point, have not cleared their infection and have bacteria still present in undetermined latent foci.4 Furthermore, of those with latent infection, it is unknown how many will subsequently have activation of infection resulting in clinical disease. The issue of whether asymptomatic infection with B. pseudomallei truly occurring during deployment of U.S. Marines to Darwin is critical for future deployments of military and civilian personnel to melioidosis-endemic locations. Melioidosis should become an important aspect of the force health protection planning. Further prospective surveillance and protocols for clinically assessing Marines with potential seroconversion are required.
Author affiliations: Austere environments Consortium for Enhanced Sepsis Outcomes, Biological Defense Research Directorate, Naval Medical Research Center, Fort Detrick, Frederick, MD (Dr. Schully, Mr. Bell, Ms. Spall, Ms. Chan, Ms. Yu, Dr. Clark, and Dr. Lawler); Department of Microbiology and Immunology, University of Nevada, Reno School of Medicine, Reno, NV (Dr. Burtnick and Dr. Brett); Global and Tropical Health Division, Menzies School of Health Research, Darwin, Australia (Mr. Mayo, Ms. Rigas, Dr. Currie); Division of Infectious Diseases, Naval Medical Center, San Diego, CA (CAPT Maves); Department of Infectious Diseases and Northern Territory Medical Program, Royal Darwin Hospital, Darwin, Australia (Dr. Currie)
Acknowledgments: Department of Defense Serum Repository, Armed Forces Health Surveillance Branch. This work was supported by work unit number A1316. The authors thank CAPT Michael Cooper from the Global Emerging Infections Surveillance section for material support and Dr. Angelia Cost from Armed Forces Health Surveillance Branch for locating the samples in the Department of Defense Serum Repository.
Disclaimer: The study protocol was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. Several of the authors of this work are military service members or employees of the United States Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that "Copyright protection under this title is not available for any work of the United States Government." Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government.
References
- Perumal Samy R, Stiles BG, Sethi G, Lim LHK. Melioidosis: clinical impact and public health threat in the tropics. PLoS Negl Trop Dis. 2017;11(5):e0004738.
- Win ZZ, Phokrai P, Aung Z, et al. Use of rapid enzyme-linked immunosorbent assay for serological screening of melioidosis in Myanmar. Am J Trop Med Hyg. 2018;98(5):1300–1302.
- Limmathurotsakul D, Golding N, Dance DA, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1(1):15008.
- Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36(1):111–125.
- Limmathurotsakul D, Kanoksil M, Wuthiekanun V, et al. Activities of daily living associated with acquisition of melioidosis in Northeast Thailand: a matched case-control study. PLoS Negl Trop Dis. 2013;7(2):e2072.
- Short BH. Melioidosis: an important emerging infectious disease—a military problem? ADF Health. 2002;3(1):13–21.
- Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4(11):e900.
- Alexander AD, Huxsoll DL, Warner AR, Jr., Shepler V, Dorsey A. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol. 1970;20(5):825–833.
- Ashdown LR, Johnson RW, Koehler JM, Cooney CA. Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J Infect Dis. 1989;160(2):253–260.
- Peacock SJ, Cheng AC, Currie BJ, Dance DA. The use of positive serological tests as evidence of exposure to Burkholderia pseudomallei. Am J Trop Med Hyg. 2011;84(6):1021–1022.
- Harris PN, Ketheesan N, Owens L, Norton RE. Clinical features that affect indirect-hemagglutination-assay responses to Burkholderia pseudomallei. Clin Vaccine Immunol. 2009;16(6):924–930.
- Cheng AC, O'Brien M, Freeman K, Lum G, Currie BJ. Indirect hemagglutination assay in patients with melioidosis in Northern Australia. Am J Trop Med Hyg. 2006;74(2):330–334.
- Wuthiekanun V, Chierakul W, Langa S, et al. Development of antibodies to Burkholderia pseudomallei during childhood in melioidosis-endemic Northeast Thailand. Am J Trop Med Hyg. 2006;74(6):1074–1075.
- Chantratita N, Wuthiekanun V, Thanwisai A, et al. Accuracy of enzyme-linked immunosorbent assay using crude and purified antigens for serodiagnosis of melioidosis. Clin Vaccine Immunol. 2007;14(1):110–113.
- Limmathurotsakul D, Chantratita N, Teerawattanasook N, et al. Enzyme-linked immunosorbent assay for the diagnosis of melioidosis: better than we thought. Clin Infect Dis. 2011;52(8):1024–1028.
- Suttisunhakul V, Wuthiekanun V, Brett PJ, et al. Development of rapid enzyme-linked immunosorbent assays for detection of antibodies to Burkholderia pseudomallei. J Clin Microbiol. 2016;54(5):1259–1268.
- Chieng S, Mohamed R, Nathan S. Transcriptome analysis of Burkholderia pseudomallei T6SS identifies Hcp1 as a potential serodiagnostic marker. Microb Pathog. 2015;79:47–56.
- Pumpuang A, Dunachie SJ, Phokrai P, et al. Comparison of O-polysaccharide and hemolysin co-regulated protein as target antigens for serodiagnosis of melioidosis. PLoS Negl Trop Dis. 2017;11(3):e0005499.
- Rubin HL, Alexander AD, Yager RH. Melioidosis—a military medical problem? Mil Med. 1963;128:538–542.
- Howe C, Sampath A, Spotnitz M. The pseudomallei group: a review. J Infect Dis. 1971;124(6):598–606.
- Clayton AJ, Lisella RS, Martin DG. Melioidosis: a serological survey in military personnel. Mil Med. 1973;138(1):24–26.
- Spotnitz M. Disease may be Vietnamese time bomb. Med World News. 1966;7:55.
- Sanford JP, Moore WL, Jr. Recrudescent melioidosis: a Southeast Asian legacy. The Am Rev Respir Dis. 1971;104(3):452–453.
- Mackowiak PA, Smith JW. Septicemic melioidosis. Occurrence following acute influenza A six years after exposure in Vietnam. JAMA. 1978;240(8):764–766.
- Mays EE, Ricketts EA. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest. 1975;68(2):261–263.
- Johnson AB, Ali N. Reactivation of latent melioidosis. Postgrad Med J. 1990;66(779):732–733.
- Koponen MA, Zlock D, Palmer DL, Merlin TL. Melioidosis. Forgotten, but not gone! Arch Internal Med. 1991;151(3):605–608.
- Kronmann KC, Truett AA, Hale BR, Crum-Cianflone NF. Melioidosis after brief exposure: a serologic survey in US Marines. Am J Trop Med Hyg. 2009;80(2):182–184.
- Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense Serum Repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92(12):1900–1904.
- Schully KL, Berjohn CM, Prouty AM, et al. Melioidosis in lower provincial Cambodia: a case series from a prospective study of sepsis in Takeo Province. PLoS Negl Trop Dis. 2017;11(9):e0005923.
- Ileri SZ. The indirect haemagglutination test in the diagnosis of melioidosis in goats. Br Vet J. 1965;121:164–170.
- Boey KW, Chan KB, Ko YC, Goh LG, Lim GC. Work stress and psychological well-being among the nursing profession in Singapore. Singapore Med J. 1997;38(6):256–260.
- Embi N, Suhaimi A, Mohamed R, Ismail G. Prevalence of antibodies to Pseudomonas pseudomallei exotoxin and whole cell antigens in military personnel in Sabah and Sarawak, Malaysia. Microbiol Immunol. 1992;36(8):899–904.
- Thin RN. Melioidosis antibodies in Commonwealth soldiers. Lancet. 1976;1(7949):31–33.
- Parameswaran U, Baird RW, Ward LM, Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009–2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196(5):345–348.
- Pang L, Harris PNA, Seiler RL, et al. Melioidosis, Singapore, 2003–2014. Emerg Infect Dis. 2018;24(1).
- Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J. The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis. 2017;11(3):e0005411.
- Samuel M, Ti TY. Interventions for treating melioidosis. Cochrane Database Syst Rev. 2002(4):CD001263.
- Parthasarathy N, Saksena R, Kovac P, et al. Application of carbohydrate microarray technology for the detection of Burkholderia pseudomallei, Bacillus anthracis and Francisella tularensis antibodies. Carbohydr Res. 2008;343(16):2783–2788.
- Charuchaimontri C, Suputtamongkol Y, Nilakul C, et al. Antilipopolysaccharide II: an antibody protective against fatal melioidosis. Clin Infect Dis. 1999;29(4):813–818.
- Anuntagool N, Wuthiekanun V, White NJ, et al. Lipopolysaccharide heterogeneity among Burkholderia pseudomallei from different geographic and clinical origins. Am J Trop Med Hyg. 2006;74(3):348–352.
- Tuanyok A, Stone JK, Mayo M, et al. The genetic and molecular basis of O-antigenic diversity in Burkholderia pseudomallei lipopolysaccharide. PLoS Negl Trop Dis. 2012;6(1):e1453.
- Burkholderia mallei. Carbohydr Res. 2013;381:6–11.
- Norris MH, Schweizer HP, Tuanyok A. Structural diversity of Burkholderia pseudomallei lipopolysaccharides affects innate immune signaling. PLoS Negl Trop Dis. 2017;11(4):e0005571.
- Tuanyok A, Mayo M, Scholz H, et al. Burkholderia humptydooensis sp. nov., a new species related to Burkholderia thailandensis and the fifth member of the Burkholderia pseudomallei complex. Appl Environ Microbiol. 2017;83(5):e02802.
- Lim YT, Jobichen C, Wong J, et al. Extended loop region of Hcp1 is critical for the assembly and function of type VI secretion system in Burkholderia pseudomallei. Sci Rep. 2015;5:8235.
- Burtnick MN, Brett PJ, DeShazer D. Proteomic analysis of the Burkholderia pseudomallei type II secretome reveals hydrolytic enzymes, novel proteins, and the deubiquitinase TssM. Infect Immun. 2014;82(8):3214–3226.
- Chang CD, Cheng KY, Jiang LX, et al. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion. 2006;46(10):1737–1744.
- Cooper A, Williams NL, Morris JL, Norton RE, Ketheesan N, Schaeffer PM. ELISA and immunopolymerase chain reaction assays for the sensitive detection of melioidosis. Diagn Microbiol Infect Dis. 2013;75(2):135–138.
- James GL, Delaney B, Ward L, Freeman K, Mayo M, Currie BJ. Surprisingly low seroprevalence of Burkholderia pseudomallei in exposed healthy adults in the Darwin region of tropical Australia where melioidosis is highly endemic. Clin Vaccine Immunol. 2013;20(5):759–760.