Tick-borne encephalitis (TBE) is a viral infection of the central nervous system that is transmitted by the bite of infected ticks, mostly found in wooded habitats in parts of Europe and Asia.1 In Germany, the rate of 0.5 confirmed cases per 100,000 people in 2019 was the third highest of the 25 European countries reporting data on TBE.1 TBE has been of historical military significance because there are a large number of U.S. service members stationed in Germany, with an estimate of about 35,000 active duty members as of September 2021.2 In the Department of Defense (DOD) reportable medical event guidelines, TBE is a notifiable event listed under arboviral diseases.3
In August 2021, the U.S. Food and Drug Administration (FDA) approved a TBE vaccine (“TICOVAC”) for U.S. travelers visiting or living in endemic areas.4 In February 2022, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted to recommend Pfizer’s TICOVAC vaccine for use in U.S. populations who travel or move to endemic areas and will have extensive exposure to ticks based on their planned outdoor activities, including many service members serving in these locations.5
A 2019 MSMR report described the cases of TBE occurring among U.S. military service members and other beneficiaries between 2006 and 2018.6 This snapshot updates these results through the end of 2021 using confirmed and probable medical event reports of TBE cases contained in the U.S. military’s Disease Reporting System internet, with a focus on the past 10-year surveillance period.
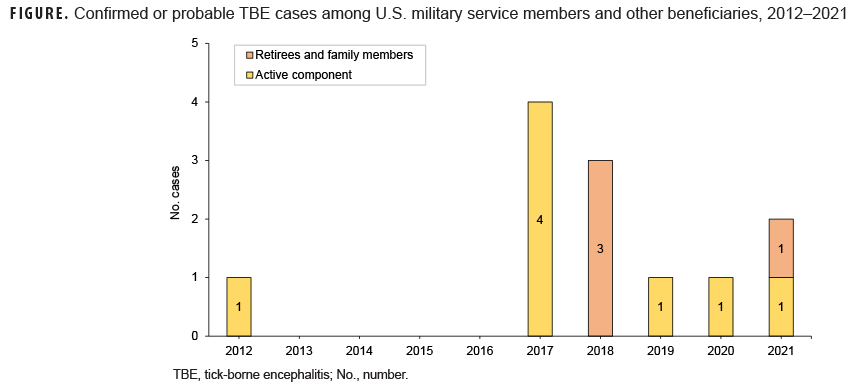
The reported TBE cases between 2012 and 2018 have been previously described,6 consisting of 1 active component service member in 2012, 4 in 2017, and 3 other beneficiaries in 2018 (Figure). In 2019, there was 1 probable case reported in a 45 year-old male Army active component service member, and in 2020 there was 1 confirmed case in a 38 year-old male Army active component service member. In 2021, there were 2 cases reported: 1 probable case in a 6 year-old female Army dependent, and 1 probable case in a 34 year-old Army active component service member. All cases reported between 2019 and 2021 occurred in Germany in the months of June and July. Case comments were available for 2 of the 4 cases; both indicated that tick exposure likely occurred from living or exercising in a wooded area. None of the cases had a prior history of TBE vaccination.
The number of TBE cases per 5-year period among military health system beneficiaries grew from 1 in 2012–2016 to 11 in 2017–2021. Although the total number of cases is small, the increase in recent years provides information that should be considered when contemplating use of the FDA-approved vaccine for U.S. service members and beneficiaries who live or participate in extensive outdoor activities in a TBE-endemic area.
References
1. European Centre for Disease Prevention and Control. Tick-borne Encephalitis – Annual Epidemiologic report for 2019. Accessed 1 April 2022. https://www.ecdc.europa.eu/en/publications-data/tick-borne-encephalitis-annual-epidemiological-report-2019
2. Defense Manpower Data Center. Number of Military and DoD Appropriated Fund (APF) Civilian Personnel Permanently Assigned By Duty Location and Service/Component. September 30, 2021. Accessed 24 March 2022. https://dwp.dmdc.osd.mil/dwp/app/dod-data-reports/workforce-reports
3. Armed Forces Health Surveillance Branch. Armed Forces Reportable Medical Events, Guidelines and Case Definitions. January 2020. Accessed 2 April 2022. https://health.mil/Reference-Center/Publications/2020/01/01/Armed-Forces-Reportable-Medical-Events-Guidelines
4. Pfizer Press Release. U.S. FDA Approves TICOVAC, Pfizer’s Tick-Borne Encephalitis (TBE) Vaccine. 13 August 2021. Accessed 1 April 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-ticovactm-pfizers-tick-borne-encephalitis
5. Pfizer Press Release. CDC Advisory Committee on Immunization Practices Votes to Recommend TICOVAC, Pfizer’s Tick-Borne Encephalitis (TBE) Vaccine, For Those at Risk of Virus Exposure. 23 February 2022. Accessed 1 April 2022. https://www.pfizer.com/news/press-release/press-release-detail/cdc-advisory-committee-immunization-practices-votes
6. Mancuso JD, Bazaco S, Stahlman S, Clausen SS, Cost AA. Tick-borne encephalitis surveillance in U.S. military service members and beneficiaries, 2006–2018. MSMR. 2019;26(11):4–10.