Complete and timely reporting of notifiable medical conditions among the Department of Defense beneficiary population is important for the control of communicable and preventable diseases and injuries. The Defense Medical Surveillance System was used to identify all hospital and ambulatory care encounters during 2018-2022 for which a notifiable medical condition was indicated among active component service members as well as all other DOD beneficiaries. Incident cases with diagnoses of DOD-notifiable medical conditions were matched with reportable medical events entered through the Disease Reporting System internet. During the study period, 61.2% of notifiable hospitalized cases and 65.5% of notifiable ambulatory care cases at a military hospital or clinic among active component service members were reported. Among other beneficiaries treated at a military hospital or clinic, only 15.2% of notifiable hospitalized cases and 22.1% of notifiable ambulatory care cases were reported to DRSi. Reporting percentages were much lower for care at outsourced facilities, regardless of the population. The timeliness of reporting for active component service members fluctuated annually, but in both 2018 and 2022, 64.2% of notifiable cases at a military hospital were reported within 1 week. For ambulatory care cases, timeliness of reporting decreased over time, with 50.1% reported within 1 week in 2018 and 43.3% in 2022.
What are the new findings?
A total of 1,306 hospitalized and 100,163 ambulatory incident cases for notifiable medical conditions among active component service members occurred from January 2018 to December 2022. Reporting of these events though the DRSi increased since the last report in 2015, but timeliness decreased. Data on other beneficiaries and outsourced care facilities were added to the analysis for this report.
What is the impact on readiness and force health protection?
Improvements to reporting and timeliness of active component service member notifiable medical conditions is needed to effectively track and mitigate communicable diseases and preventable injuries. Although reporting currently is not required for all non-service member beneficiaries, they present another source of communicable diseases that could affect disease among service members, making them a potential population for report consideration.
Background
U.S. military Services are required, by Department of Defense Directive 6490.02E, to report notifiable medical conditions among all DOD personnel and dependents, residing either in garrison or overseas with their military sponsor, during a possible exposure or public health event.1 Centralized reporting of preventable and communicable medical conditions is an important tool for facilitating rapid dissemination of information on the occurrence of medical events that could pose a public health threat. Centralized reporting also serves as a mechanism for recording more extensive information about a medical event in hospitalization and ambulatory care administrative data. The guidelines and specific case definitions for all medical conditions required by DOD for reporting are described in Armed Forces Reportable Medical Events: Guidelines and Case Definitions.2 Currently, all services report notifiable medical conditions through a single electronic system, the Disease Reporting System Internet, accessible at all military hospitals and clinics.3
The usefulness of these reportable medical events is highly dependent on the completeness and timeliness of their submission. For optimally-informed determinations by military leadership and public health officers about the locations and extents of possible outbreaks, all cases of notifiable medical conditions need to be reported, with all required information, as soon as possible. During the COVID-19 pandemic, DRSi became a key data source for tracking confirmed cases of COVID-19.4 Reports on preventable notifiable medical conditions such as heat and cold injuries are produced frequently to assist the evaluation and potential modification of prevention strategies. The Armed Forces Health Surveillance Division of the Defense Health Agency publishes communicable disease RMEs in each issue of the MSMR.
The most recent analysis of the completeness and timeliness of RMEs, published in the MSMR in November 2015, only reported on active component service members.5 The 2008-2014 analysis found that 47.6% of notifiable hospitalized cases and 57.2% of notifiable ambulatory cases were reported as RMEs to DRSi. Since the 2015 report on 2008-2014 data, medical encounters are now coded with ICD-10 diagnostic codes, which provide better specificity than ICD, 9th revision diagnostic codes and improve identification of health conditions.6 Three revisions to DOD reporting guidelines since 2015 aligned laboratory and clinical criteria with standardized case definitions published by the Nationally Notifiable Disease Surveillance System, which added or removed RMEs.7 This report estimates RME completeness and timeliness from 2018 to 2022 among ACSM and all other DOD beneficiaries.
Methods
The Defense Medical Surveillance System contains administrative records for all medical encounters of DOD beneficiaries who were hospitalized or received ambulatory care at military hospitals and clinics, or for civilian Click to closePurchased CareThe TRICARE Health Program is often referred to as purchased care. It is the services we “purchase” through the managed care support contracts.purchased care at an outsourced care facility. Records of health care encounters from both sources of care were included in this analysis, but analyzed separately. Hospitalizations and ambulatory encounters for all DOD beneficiaries between January 1, 2018 and December 31, 2022 were searched for diagnoses of notifiable medical conditions in the primary diagnostic position using the 10th revision of International Classification of Diseases diagnostic codes listed in Table 1.
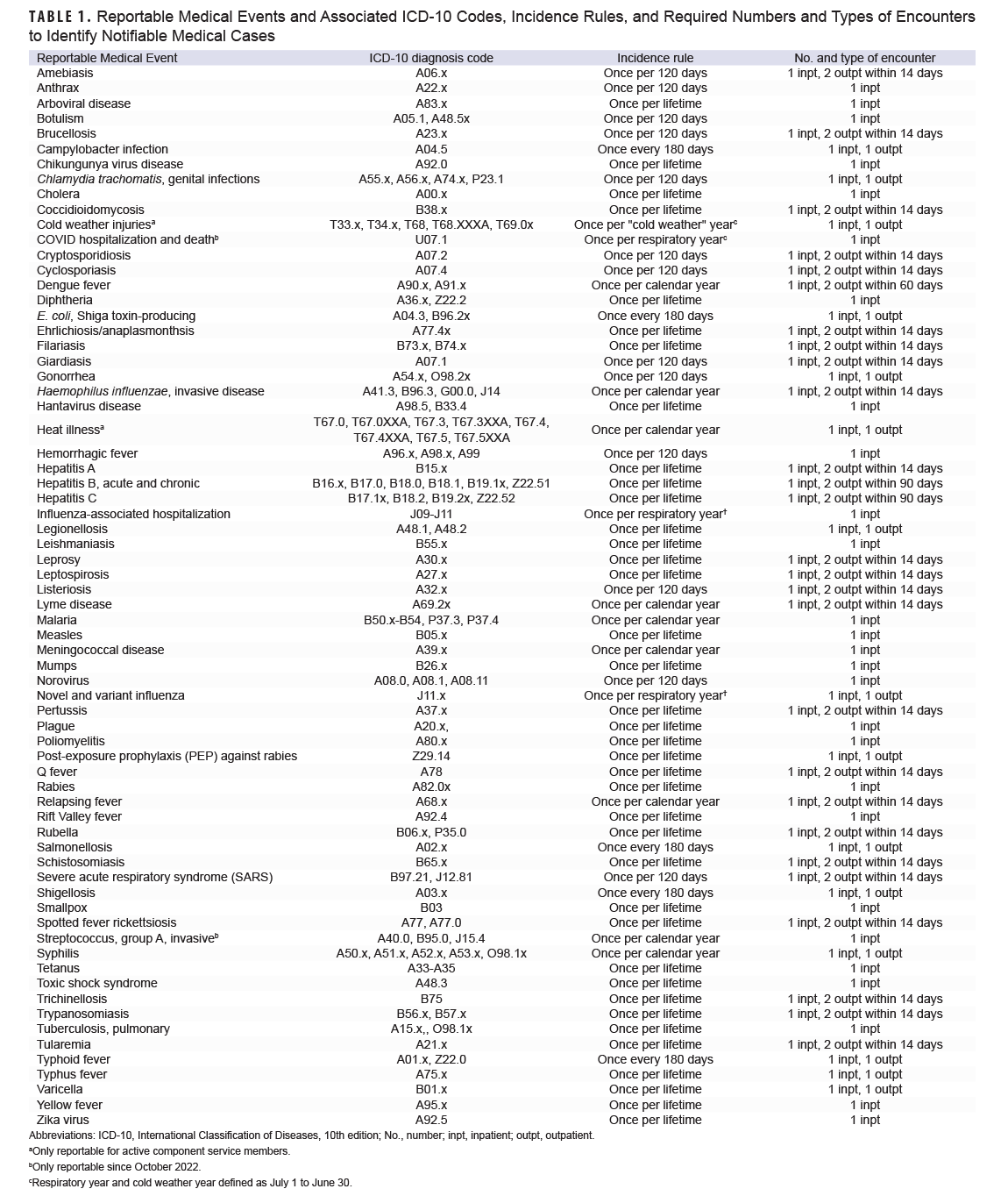
Potentially notifiable cases were identified by the required numbers and types of encounters delineated in Table 1 for specified conditions. For example, a potential case of amebiasis would require an ICD-10 code of A06.x in the primary diagnostic position in the record of a hospitalization, or 2 ambulatory encounters within 2 weeks. Incident cases of the various conditions for individuals were then identified by applying the incidence rule listed for each condition. For example, an individual who met the criteria for a potentially notifiable case of amebiasis in February 2019 but was previously diagnosed with amebiasis in December 2018 (within 120 days of the February 2019 diagnosis) would not be counted as a newly incident case in 2019 (Table 1).
The DMSS also contains RME records entered in DRSi for all DOD beneficiaries. RMEs from event dates January 1, 2018 through December 31, 2022 were identified from DMSS for DOD beneficiaries with an incident notifiable medical condition hospitalization or ambulatory encounter. By notifiable medical condition, RMEs were matched to incident hospitalization or ambulatory encounters. If more than 1 RME for the specific condition was found, the RME with an event date closest to the encounter event date was selected. The total number of incident notifiable medical conditions and the percentage with a corresponding RME were calculated by population (ACSM or other beneficiaries), source of care (military hospital/clinic or OCF), RME category, and year. The “other beneficiary” category included service members in the reserve or guard components, retirees, and dependents. Reporting timeliness was estimated by calculating the number of weeks between the incident case’s medical encounter or hospitalization date and the RME entry date in DRSi. Among the incident notifiable medical cases matched to an RME, the percentages that were reported within 1 week, 2 weeks, and 4 weeks were calculated. All data were analyzed using SAS Enterprise Guide v8.3 Update 3 (SAS Institute, Cary, NC).
Results
Active Component Service Members
During the study period, 61.2% of incident notifiable hospitalized medical cases among ACSM seen at a military hospital were reported as RMEs, ranging from a low in 2020 (56.0%) to a high in 2022 (71.1%). Reporting percentages for hospitalized cases at OCFs were much lower than at military hospitals, with an overall reporting of 18.1% and a range of 7.9% (2020) to 24.4% (2019) (Table 2). Table 2 also shows the percentage of incident notifiable ambulatory medical cases reported as RMEs. Over the study period, 65.5% of incident notifiable ambulatory medical cases at military clinics among ACSM were reported as RMEs, substantially higher than the percent of ambulatory medical cases reported from OCFs (11.9%).
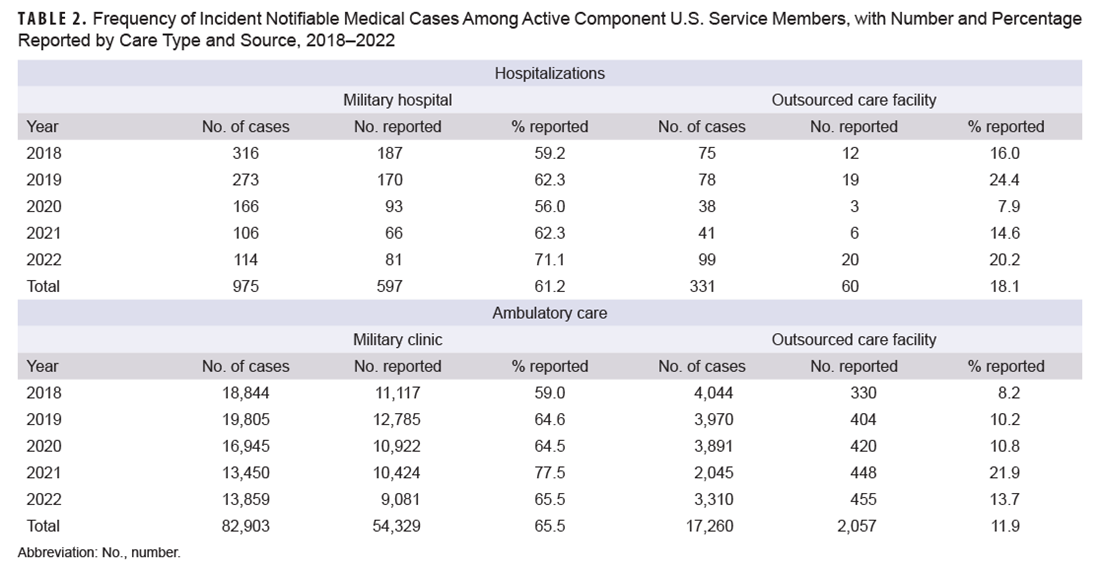
The total numbers of incident cases for each type of notifiable medical condition among active ACSM at military hospitals and clinics are shown in Table 3. For hospitalized conditions, the largest numbers of incident cases were for heat illness (368 cases), influenza-associated hospitalizations (128 cases), and malaria (65 cases). Of those, 67.7% of heat illness cases, 40.6% of influenza-associated hospitalization cases, and 87.7% of malaria cases were reported as RMEs. The most frequent incident ambulatory conditions were chlamydia (43,673 cases, 91.3% reported), novel and variant influenza (16,083 cases, 0.03% reported), and heat illness (6,915 cases, 48.2% reported).
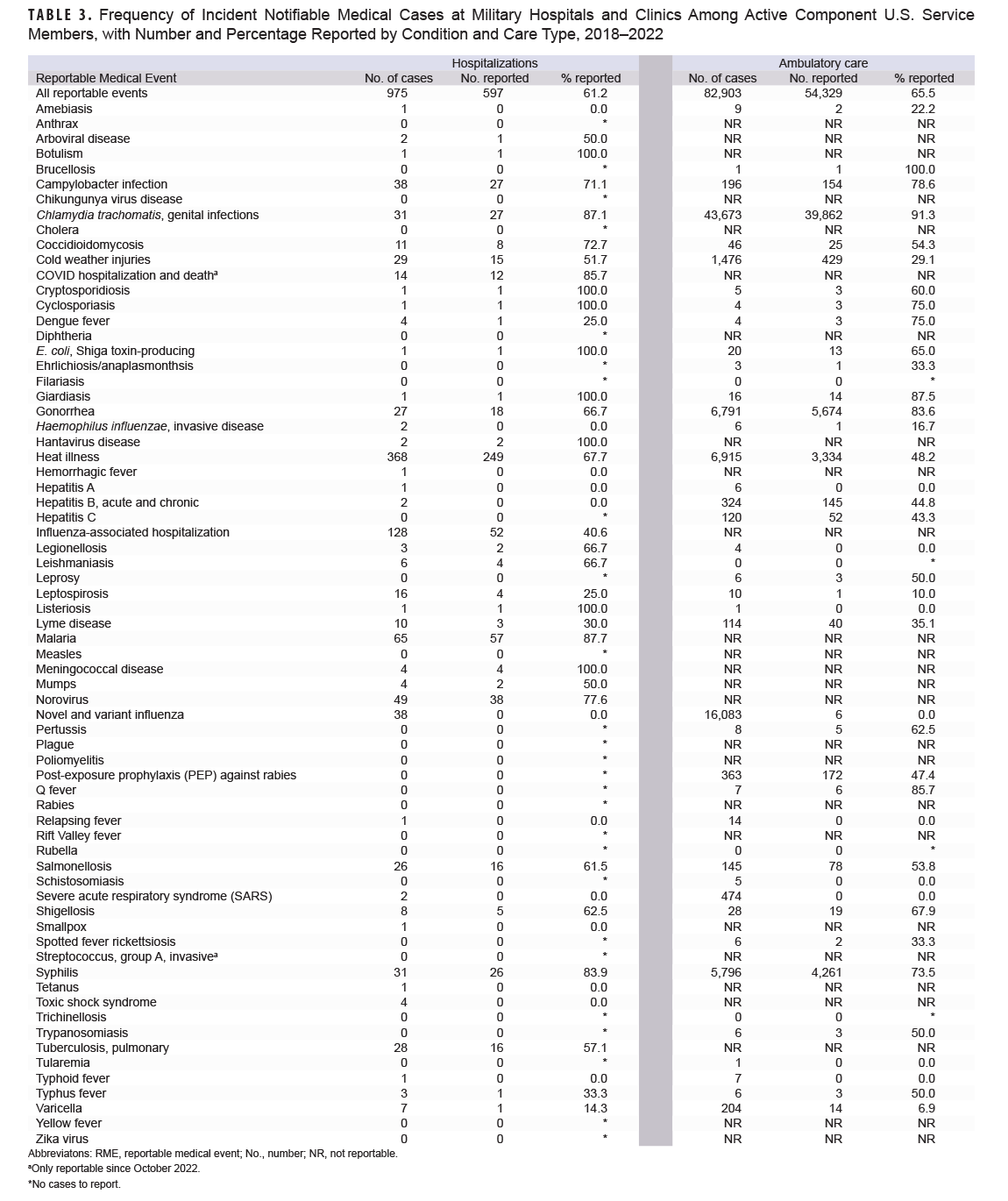
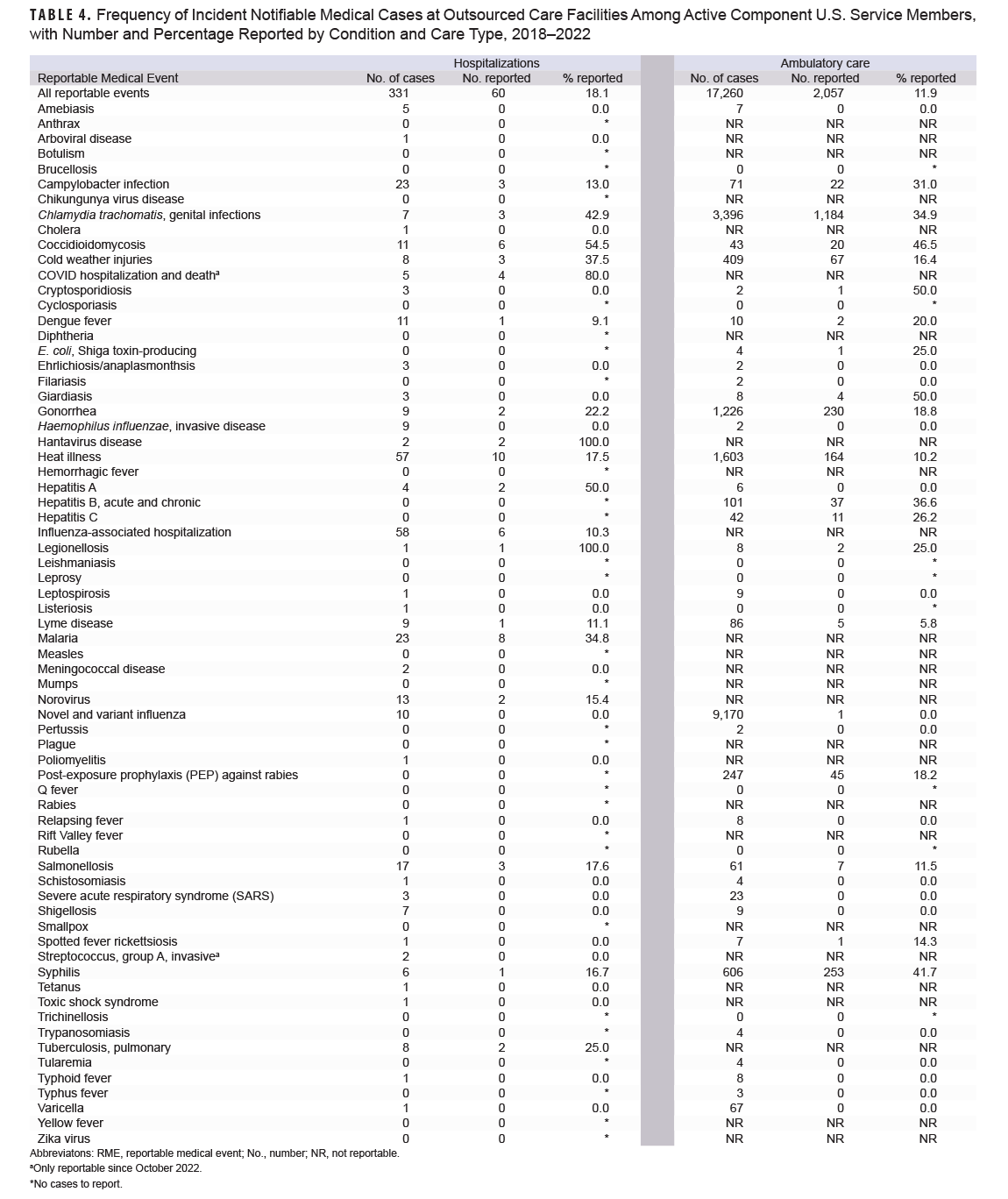
Table 4 presents the notifiable medical conditions at OCFs among ACSM. Although much lower in magnitude, hospitalizations at OCFs had similar trends in condition frequency compared to military hospitals and clinics. The most frequently hospitalized medical conditions at OCFs were influenza-associated (58 cases, 10.3% reported), heat illness (57 cases, 17.5% reported), malaria (23 cases, 34.8% reported), and campylobacter infection (23 cases, 13.0% reported). Among ambulatory conditions reported at OCFs, novel and variant influenza (9,170 cases, 0.01% reported), chlamydia (3,396 cases, 34.9% reported), and heat illness (1,603 cases, 10.2% reported) were the most frequent.
The timeliness of reporting of incident notifiable medical conditions among ACSM varied by care type and facility (Table 5). Between 2018 and 2022, timeliness of notifiable conditions for military hospitalizations reported within one week to DRSi ranged from 48.5% to 64.2%; this range increased to 91.8% and 98.9% when evaluating timeliness within one month. Regardless of facility type, ambulatory care reporting timeliness to DRSi within one week decreased over time, declining from 40.5% in 2018 to 35.1% in 2022 at military clinics and 26.4% to 17.6% for OCFs. OCF hospitalizations was the only group to improve in reporting timeliness over the study period, with reporting within one week rising from 25.0% in 2018 to 40.0% in 2022.
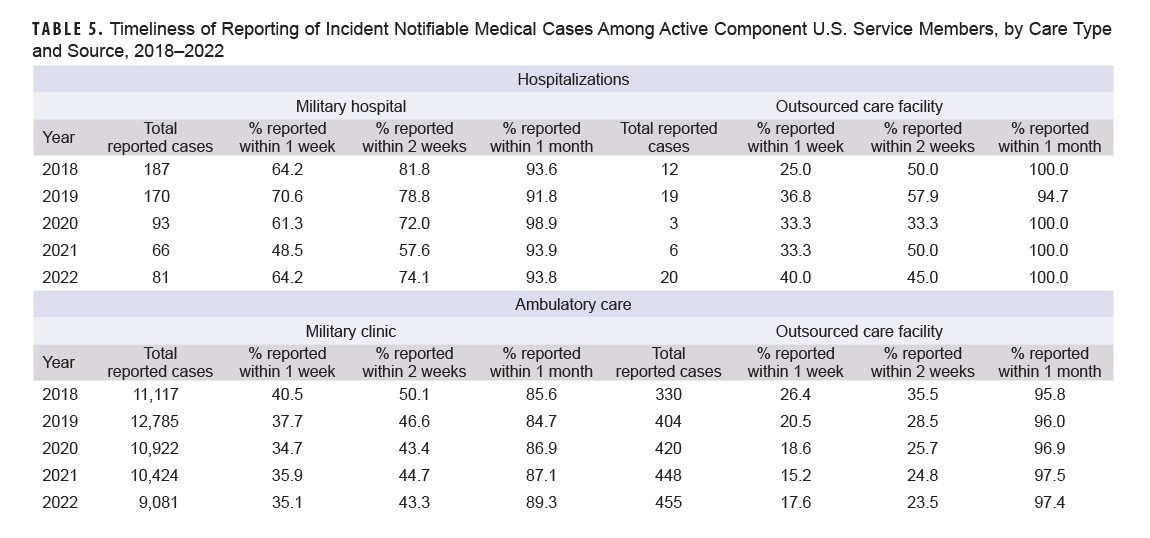
Other Beneficiaries
During the surveillance period, only 15.2% of incident notifiable hospitalized medical cases among other beneficiaries seen at military hospitals were submitted as RMEs (Table 6). Reporting increased from 8.8% in 2018 to 25.5% in 2021 but dropped to 18.0% in 2022. Reporting for hospitalizations at OCFs were significantly lower than at military hospitals and clinics, with an overall reporting of 0.9% and range of 0.5% (2022) to 2.0% (2021) (Table 6). Table 6 also provides the percentage of incident notifiable ambulatory care medical conditions submitted as RMEs. During the study period, the military clinic reporting percentage for ambulatory conditions among other beneficiaries was higher than for hospitalized conditions (22.1% vs. 15.2%). Reporting of ambulatory conditions at OCFs was very low (0.5%).
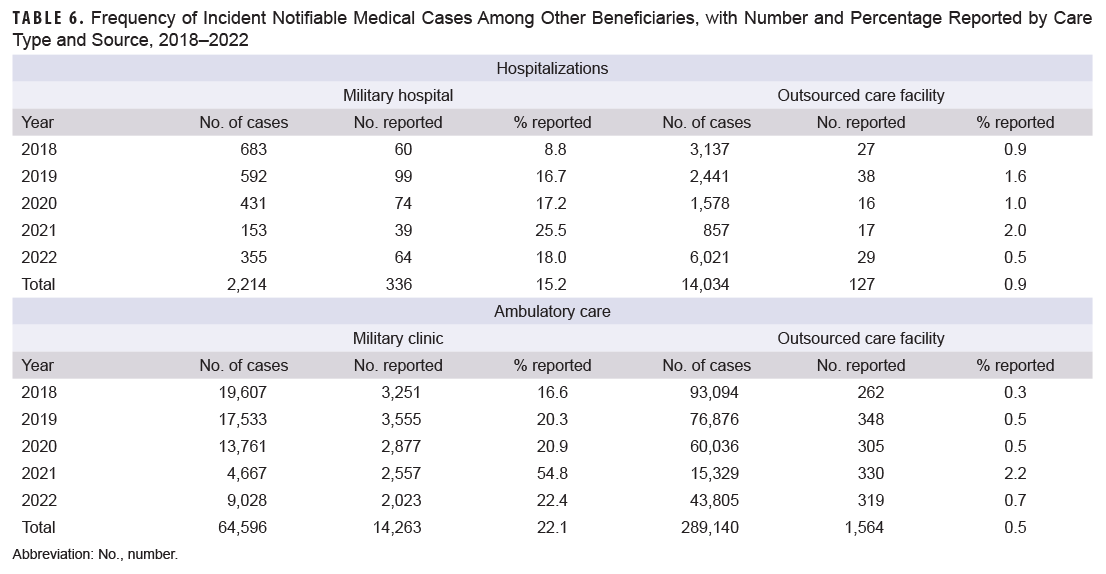
The total numbers of incident cases for each type of notifiable medical condition among other beneficiaries at military hospitals or clinics are shown in Table 7. For hospitalized conditions during 2018-2022, the largest numbers of incident cases were influenza-associated (928 cases), novel and variant influenza (400 cases), and COVID hospitalization and death (only reportable in 2022; 169 cases). Of those cases, 11.7% of influenza-associated hospitalizations, 0.0% of novel and variant influenza cases, and 16.6% of COVID hospitalizations and death were submitted as RMEs. The most frequent incident ambulatory conditions were novel and variant influenza (42,184 cases, 0.05% reported), chlamydia (13,397 cases, 79.7% reported), and gonorrhea (2,437 cases, 69.8% reported).
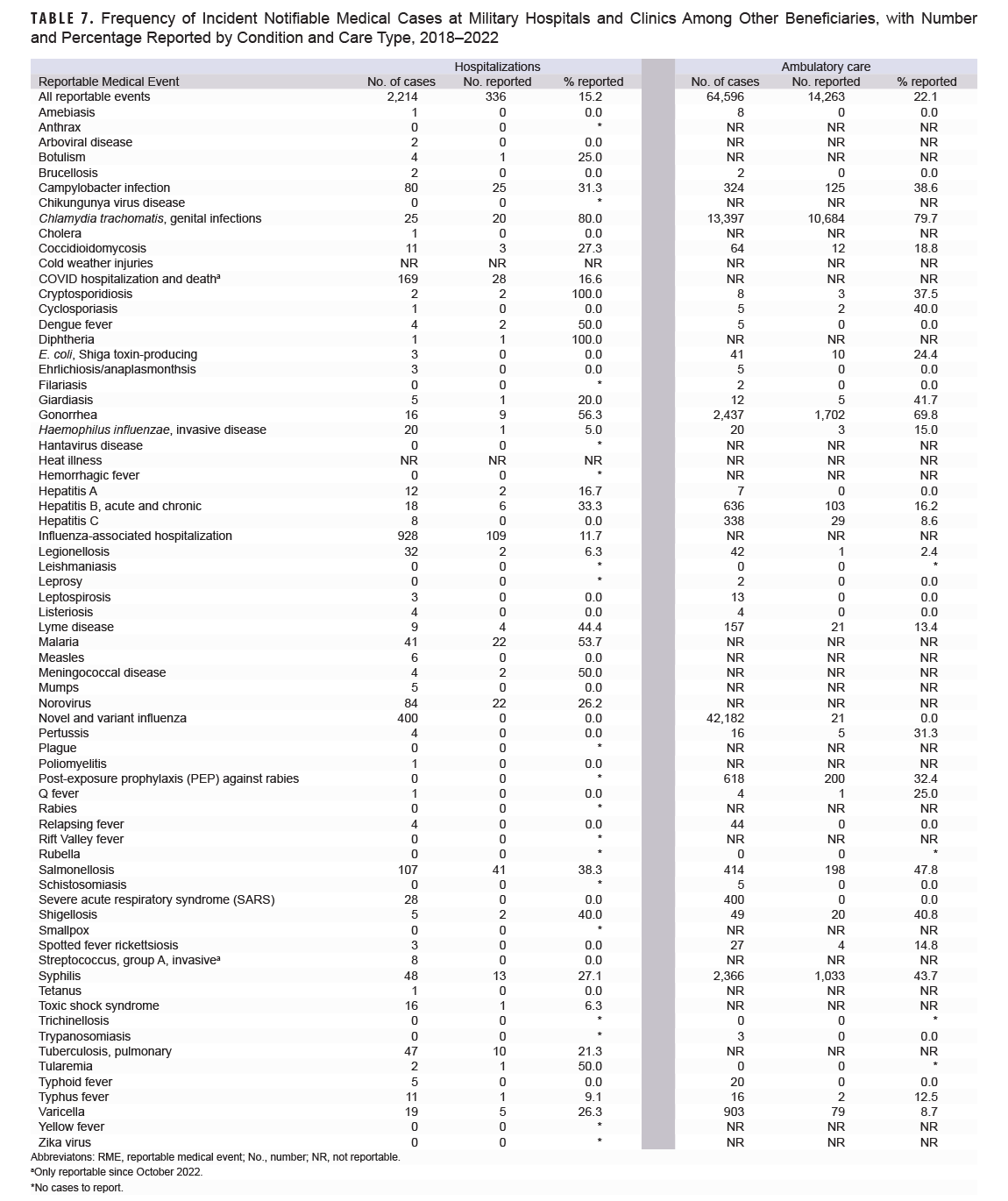
Table 8 lists the notifiable medical conditions among other beneficiaries treated at an OCF. The most frequent hospitalized medical conditions were 5,061 influenza-associated cases (0.45% reported), 3,785 cases of COVID hospitalizations and death (only reportable in 2022; 0.3% reported), and 698 cases of salmonellosis, of which 3.0% were submitted as RMEs. Among ambulatory care conditions, the most frequent were novel and variant influenza (238,111 cases, <0.01% reported), chlamydia (17,890 cases, 5.0% reported), and gonorrhea (6,788 cases, 2.6% reported).
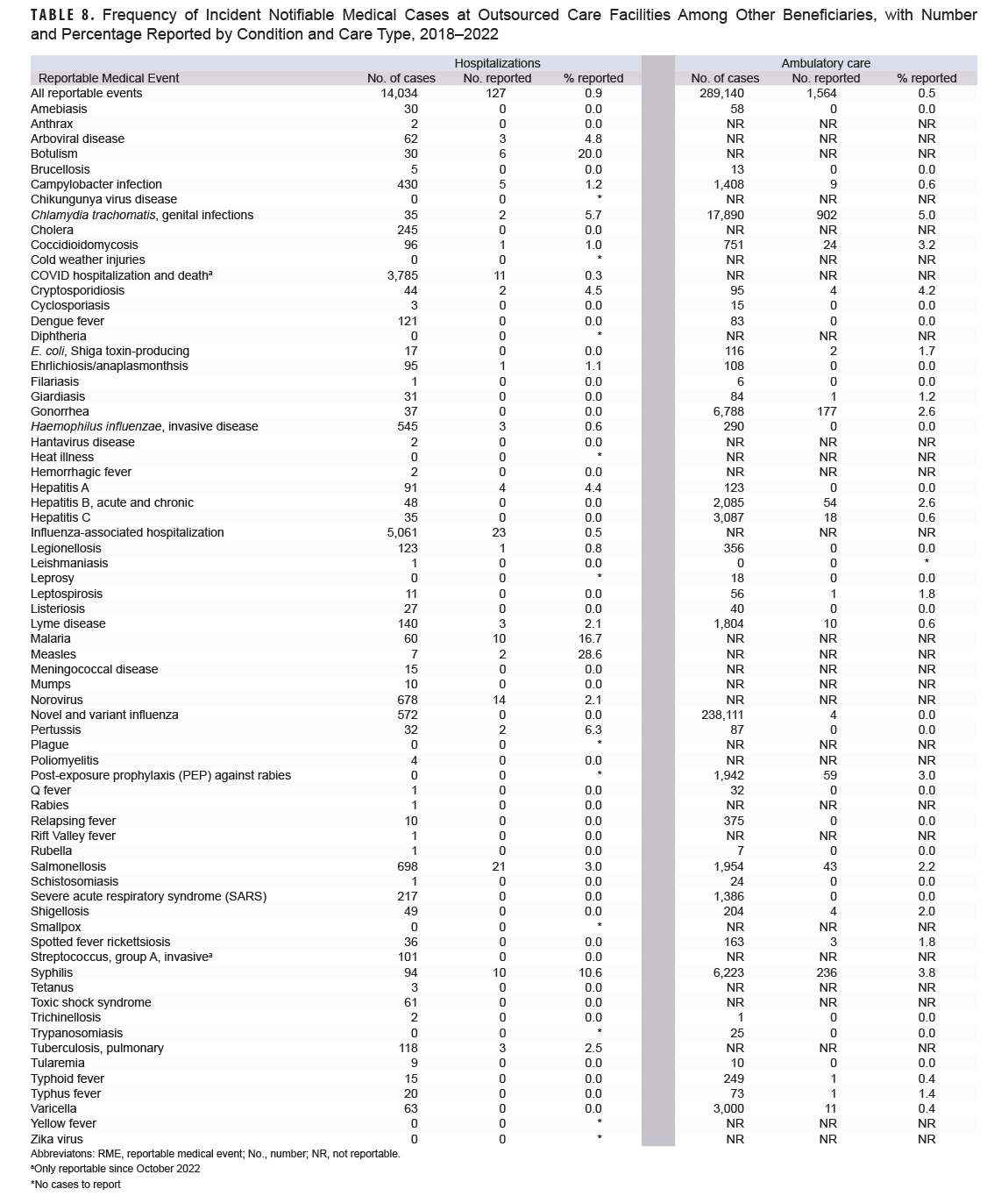
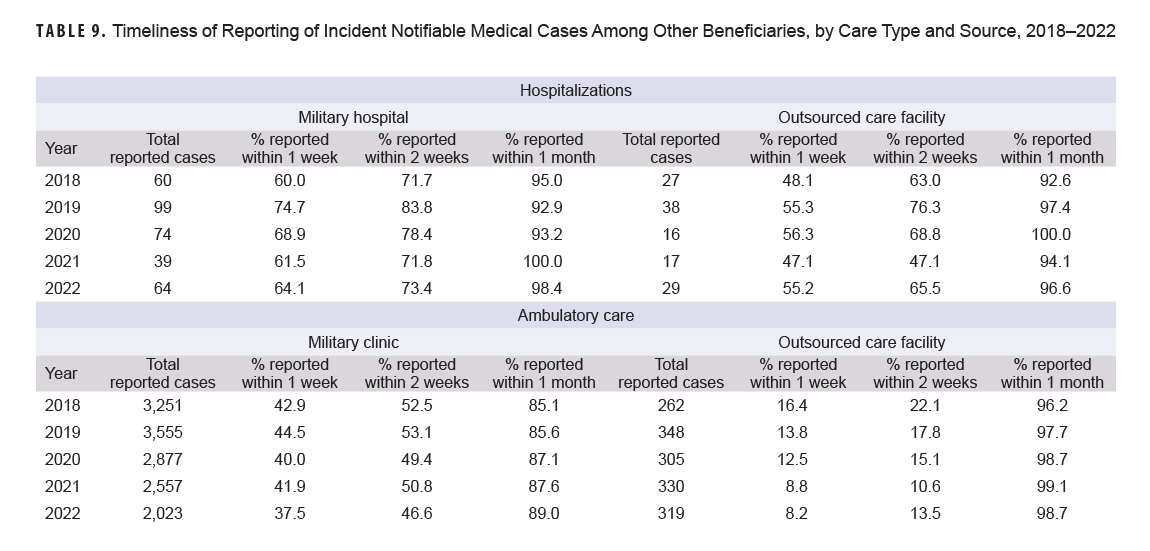
The timeliness of reporting incident hospitalized notifiable medical conditions among other beneficiaries fluctuated annually, but increased overall from 2018 to 2022 for both military hospitals and OCFs (Table 9). Between 2018 and 2022, hospitalization reporting within one week rose from 60.0% to 64.1% for military care and from 48.1% to 65.6% for OCF care. Regardless of facility type, reporting timeliness for ambulatory care within one week decreased, from 42.9% in 2018 to 37.5% in 2022 for military care and from 16.4% to 8.2% for OCFs.
Discussion
The results of this analysis indicate that in recent years just over 60% of ACSM with incident hospitalized notifiable medical conditions identified at a military hospital were reported as RMEs through the DRSi reporting system. Case reporting of notifiable medical conditions identified from ambulatory encounters at military clinics was slightly higher (65.5%) than cases identified from hospitalization records. To compare these results to the 2015 analysis published in the MSMR,5 active component case reporting from both military hospitals and clinics as well as OCFs were combined, resulting in reporting estimates of 50.3% and 56.3% for hospitalized and ambulatory cases, respectively. These findings show an increase in reporting from the 2015 analysis, which found that 46.7% of hospitalized cases and 55.2% of ambulatory cases were reported in 2014.5
This first analysis of RME reporting among other DOD beneficiaries reveals a significantly lower number of incident notifiable medical cases reported among other beneficiaries compared to ACSM. Approximately one-fifth of cases among other beneficiaries from military clinics and hospitals were reported to DRSi, and only a negligible proportion from OCFs were reported. Because DOD Directive 6490.02E specifies health surveillance activities for dependents residing in garrison or overseas with their military sponsor during a possible exposure or public health event, the low proportion of incident notifiable medical cases reported among other beneficiaries was expected.1 In addition, because personnel resources for public health reporting may be limited at certain facilities, active component service member case reporting will take precedence over conditions from other beneficiaries, especially considering the greater number of hospitalizations among the other beneficiary population. Despite the fact there is no requirement for reporting of notifiable medical events for all dependents, this analysis was conducted to demonstrate the utility of RME reports for the larger MHS beneficiary population.
The timeliness of reporting for ACSM fluctuated over the 5-year surveillance period. Notifiable case reporting within one week from military hospitals and clinics ebbed from 2020 to 2021. This report does not speculate on factors influencing slower RME reporting, which are likely multifactorial and may include the COVID-19 pandemic. Throughout the surveillance period, however, the timeliness of reporting for hospitalized active component cases within one month remained above 90%. For active component cases identified from military clinic ambulatory care, timeliness of reporting within one month increased, approaching 90% (89.3%) by 2022.
Interpretation of these findings should be with caution due to methodological limitations. First, use of administrative data from health records to identify cases warranting RME submission likely overestimates the number of true cases of the conditions of interest, which is especially true for preliminary outpatient diagnoses, which do not meet RME criteria. Laboratory test results that are inconclusive or pending negative do not meet RME criteria. Conversely, common diagnoses such as heat and cold injuries are based on clinical findings at a single ambulatory encounter and do not require laboratory confirmation, and these diagnoses produced RME submissions for only one-third of cold injury and half of heat injury outpatient cases identified.
The data available for this analysis do not permit any insight into subsequent exclusion of preliminary diagnoses of such conditions when considering RME submission. Because many reportable conditions that may be of critical importance for rapid public health response are so infrequently encountered, clinical suspicion may result in diagnosis documentation prior to confirmation. It would not be surprising that many, if not most, such tentative diagnoses would fail to meet RME criteria.
Outsourced care providers are not required to report to the DOD, nor do they have access to DRSi, so reporting of outsourced care encounters for notifiable medical conditions relies on the referring military hospital or clinic to follow up and report, or for public health reporting personnel to identify these cases in the medical record. These additional steps for reporting likely contributed to the low OCF reporting numbers and present a missed opportunity.
This analysis does not include RMEs without a matching incident hospitalization or clinic encounter, and therefore timeliness of non-matching RMEs could not be assessed, nor are the total RMEs in this analysis the complete number of RMEs for the surveillance period.
One of the most frequently diagnosed conditions during ambulatory care, regardless of population or source of care, was novel and variant influenza. Although ambulatory seasonal influenza is not a reportable condition, novel and variant influenza is reportable. Given that influenza surveillance data for the U.S. during the study period do not indicate expansive circulation of novel or variant influenza strains, it is likely encounters for seasonal influenza were miscoded,8,9 which is further supported by the finding that only a few of these encounters were reported.
Complete and timely reporting of notifiable medical conditions is an important factor for public health prevention and control of communicable disease and injuries. These findings indicate that improvements are needed for complete and timely reporting among ACSM. Although reporting is not currently required for non-DOD personnel, the large number of notifiable medical conditions in this population present a potential transmission risk to service members, and consideration should be given to tracking notifiable medical conditions in this population as well. Revisions to RME guideline training and corresponding enhancements among public health personnel to increase accuracy of coding for medical encounters could produce needed improvements in RME reporting and timeliness.
References
- Department of Defense. DOD D6490.02E, Comprehensive Health Surveillance. Accessed on Oct. 2, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodd/649002Ep.pdf?ver=2019-04-08-104448-613Armed Forces Health Surveillance Center. Armed Forces Reportable Medical Events: Guidelines and Case Definitions. Accessed Oct. 2, 2023. https://www.health.mil/Reference-Center/Publications/2022/11/01/Armed-Forces-Reportable-Medical-Events-Guidelines
- Navy and Marine Corps Public Health Center. DRSi: Disease Reporting System Internet. Accessed Oct. 2, 2023. https://www.med.navy.mil/Navy-and-Marine-Corps-Force-Health-Protection-Command/Preventive-Medicine/Program-and-Policy-Support/Disease-Surveillance/DRSI
- Kebisek J, Maule A, Smith J, et al. Integration of multiple surveillance systems to track COVID-19 in the U.S. Army Population. Mil Med. 2021;188(7-8):e2583-e2591.
- Hurt L, Ying S. Completeness and timeliness of reporting of notifiable medical conditions, active component, U.S. Armed Forces, 2008-2014. MSMR. 2015;22(11):8-21.
- United States Department of Health and Human Services, Centers for Disease Control and Prevention. National Center for Health Statistics. International Classification of Diseases (ICD-10-CM/PCS) Transition–Background. Accessed Oct. 6, 2023. https://www.cdc.gov/nchs/icd/icd10cm_pcs_background.htm
- United States Department of Health and Human Services, Centers for Disease Control and Prevention. National and Notifiable Disease Surveillance System: Surveillance Case Definitions for Current and Historical Conditions. Accessed Oct. 4, 2023. https://ndc.services.cdc.gov
- United States Department of Health and Human Services, Centers for Disease Control and Prevention. Past Weekly Surveillance Reports. Oct. 2, 2023. https://www.cdc.gov/flu/weekly/pastreports.htm
- Defense Health Agency, Armed Forces Health Surveillance Division. Influenza Summary and Reports. Accessed Oct. 2, 2023. https://www.health.mil/Military-Health-Topics/Health-Readiness/AFHSD/Reports-and-Publications/Influenza-Summary-and-Reports