Abstract
Infectious mononucleosis (IM) is an acute infectious illness characterized by swollen lymph nodes, fever, pharyngitis, fatigue, and head and body aches. This report describes the incidence rates, trends, and demographic correlates of IM among active component service members during 2002–2018. During the surveillance period, there were 23,780 incident cases of IM, resulting in an overall incidence rate of 104.2 cases per 100,000 person-years (p-yrs). The incidence of IM diagnoses was highest among the youngest age groups and decreased with increasing age. The rate of incident IM diagnoses was markedly higher among non-Hispanic white service members (123.4 per 100,000 p-yrs) compared to those in other race/ethnicity groups. The incidence of IM diagnoses among recruits (364.9 per 100,000 p-yrs) was 3.4 times that among other enlisted personnel (106.0 per 100,000 p-yrs) and 5.6 times that among officers (64.7 per 100,000 p-yrs). The incidence of IM diagnoses remained relatively stable during the surveillance period, at about 100 per 100,000 p-yrs. IM is not considered to be a serious illness; however, it can seriously impact availability for duty during the acute phase.
What Are the new Findings?
An average of 1,398 service members per year were diagnosed with IM during 2002–2018. Incidence rates were highest among the youngest age groups, recruit trainees, females, non-Hispanic whites, and health care workers. The crude overall incidence of IM diagnoses was 104.2 per 1,000 p-yrs; annual rates remained relatively stable over the 17-year surveillance period.
What Is the Impact on Readiness and Force Health Protection?
Symptomatic cases of IM can result in 2 weeks or more of limited duty, resulting in 2,797 weeks or more of lost duty time per year. Recruit trainees with IM may need to be recycled. Cases with possible splenic enlargement may be cautioned or prevented from strenuous physical activities for up to 4 weeks after onset of IM.
Background
Infectious mononucleosis (IM) is an acute infectious illness characterized by swollen lymph nodes, fever, pharyngitis, fatigue, and head and body aches. Less common but more severe manifestations may include swelling of the liver or spleen.1 It is estimated that at least 90% of cases of IM are caused by Epstein-Barr virus (EBV), but mononucleosis-like illnesses can be caused by other pathogens (e.g., cytomegalovirus, human herpes virus 6, human immunodeficiency virus type 1, Toxoplasma gondii).2,3 Acute symptoms usually present within 4 to 6 weeks after infection with EBV and generally resolve within 2 to 4 weeks; however, fatigue and poor functional status may persist for months.1 Rare acute complications include splenic rupture, hepatitis, and respiratory tract or nasopharyngeal obstruction (e.g., tonsillar enlargement).4 Prospective studies suggest that 9–12% of adults with IM go on to meet the criteria for chronic fatigue syndrome 6 months following IM onset; however, no definitive causal relationship between IM and chronic fatigue syndrome has been determined.5–8
Viruses that cause IM are most commonly transmitted through saliva but can also be transmitted through other bodily fluids, including blood and semen.1 EBV may be shed in salivary secretions at high levels for a prolonged period following clinical recovery, and the virus may be intermittently shed at lower levels in the oropharynx for decades.9–11 Most people infected with IM will experience symptoms only once. However, reactivation of EBV may occur later in life in those who are immunocompromised (e.g., those who have had an organ transplant, those who are using immunosuppressive medication, or those with acquired immune deficiency syndrome).12 Infection with EBV is very common worldwide, and it is estimated that 90% of adults are antibody positive before age 30.2 Risk of acquiring IM is highest in populations of young adults who function or live in close proximity to one another, such as students or military service members.13
In the U.S., it is estimated that almost 90% of 18–19-year-olds test positive for EBV antibody.11 In addition, studies have shown that the incidence of IM can range between 11 and 48 cases per 1,000 persons per year among students and military service members.14 A previous MSMR analysis found an incidence of IM of 98.9 per 100,000 person-years (p-yrs) among active component service members.15 The incidence of IM was higher among females, whites, younger individuals, and those in the Air Force and Navy, compared to their respective counterparts.15
Because IM is common among young adults living in close proximity and because it has a relatively long duration of symptoms, IM has the potential to reduce military operational readiness by contributing to lost or limited duty time. The purpose of this report is to describe the overall incidence rates, trends, morbidity and health care burden, and demographic correlates of IM among active component service members during 2002–2018.
Methods
The surveillance period was 1 Jan. 2002 through 31 Dec. 2018. The surveillance population included all active component service members who served in the Army, Air Force, Navy, or Marine Corps at any time during the surveillance period. All data used for analyses were abstracted from records routinely maintained in the Defense Medical Surveillance System (DMSS) for health surveillance purposes.
For the incidence analysis, an incident case of IM was defined by having a qualifying diagnosis (International Classification of Diseases, 9th edition [ICD-9]: 075; International Classification of Diseases, 10th edition [ICD-10]: B27*) in the first diagnostic position of an inpatient or outpatient medical encounter. An individual was counted as an incident case only once per lifetime. Prevalent cases (i.e., incident cases that occurred before the start of the surveillance period) were removed from the incidence analysis, and person-time was censored at the time of the incident diagnosis. If a service member had both an outpatient and an inpatient case-defining medical encounter on the same day, the inpatient diagnosis was prioritized over the outpatient diagnosis. Crude incidence rates of IM were calculated as incident IM diagnoses per 100,000 p-yrs.
Adjusted incidence rates of IM were calculated by certain military demographic characteristics (i.e., service branch, occupation, and rank/grade) using multivariable Poisson regression models. One model was run for each military demographic characteristic and adjusted for age, sex, and race/ethnicity. Adjusted incidence rates were calculated per 100,000 p-yrs.
To assess the prevalent burden of IM in the Military Health System (MHS), the total numbers of medical encounters and hospital bed days occurring during the surveillance period with a diagnosis of IM in the primary diagnostic position were counted using standard MSMR burden methodology as were the numbers of individuals affected.16 These burden counts included encounters for both prevalent and incident cases of IM.
Results
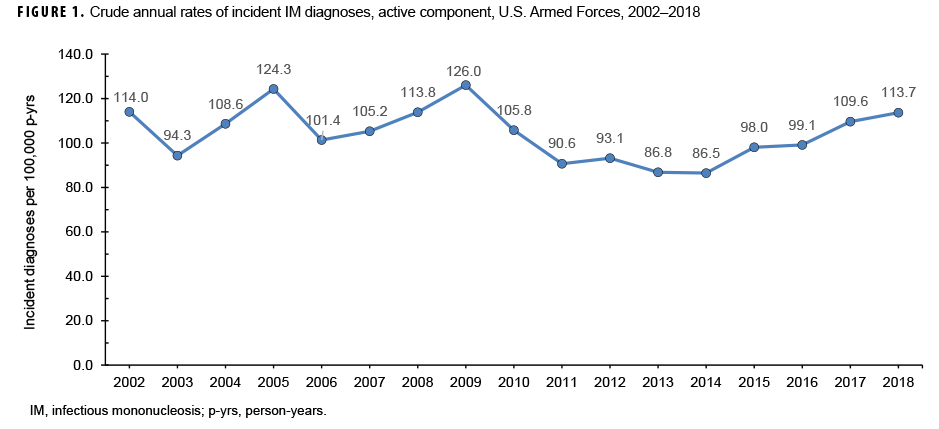
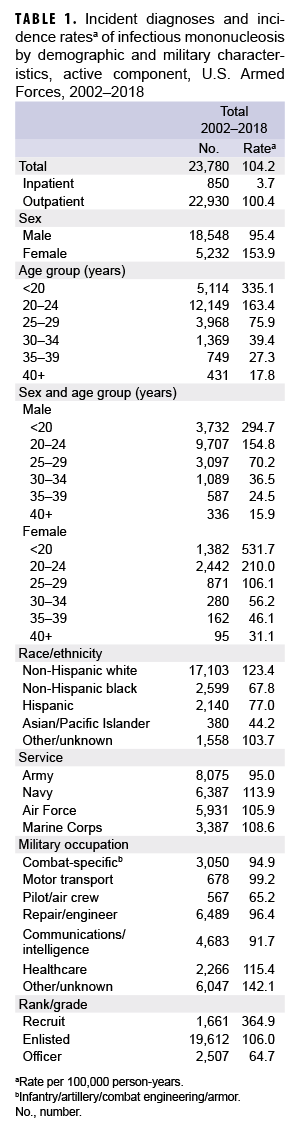
During the 17-year surveillance period, there were 23,780 incident cases of IM, resulting in a crude (unadjusted) overall incidence rate of 104.2 cases per 100,000 p-yrs (Table 1). The vast majority (96.4%) of incident cases were diagnosed in outpatient settings. The crude annual rate of incident IM diagnoses fluctuated from a low of 86.5 per 100,000 p-yrs in 2014 to a high of 126.0 per 100,000 p-yrs in 2009 (Figure 1). However, the incidence of IM diagnoses at the beginning of the surveillance period in 2002 (114.0 per 100,000 p-yrs) was very similar to the incidence at the end of the surveillance period in 2018 (113.7 per 100,000 p-yrs).
Overall incidence rates of IM diagnoses were highest among the youngest age groups and decreased with increasing age (Table 1). Among female service members, the overall incidence of IM diagnoses among women less than 20 years old (531.7 per 100,000 p-yrs) was 17.1 times that among women in the oldest age group (40 years or older; 31.1 per 100,000 p-yrs). Similarly, among male service members, the overall rate of incident IM diagnoses among those less than 20 years old (294.7 per 100,000 p-yrs) was 18.5 times that of men aged 40 years or older (15.9 per 100,000 p-yrs). Overall, the incidence of IM diagnoses was 61.3% higher in females compared to males (153.9 per 100,000 p-yrs vs. 95.4 per 100,000 p-yrs, respectively). In addition, the overall rate of incident IM diagnoses was markedly higher among non-Hispanic white service members (123.4 per 100,000 p-yrs) compared to those in other race/ethnicity groups.
The crude overall incidence rates of IM diagnoses were generally similar across the service branches; however, the rate was lowest in the Army (95.0 per 100,000 p-yrs) and highest in the Navy (113.9 per 100,000 p-yrs). Service members working in health care had the highest overall rate (115.4 per 100,000 p-yrs) among the specific occupational categories. The high rate for those in the non-specific category of "other/unknown" was largely due to the fact that 37% (n=2,238) of the cases were among recruit trainees. The overall incidence of IM diagnoses among recruits (364.9 per 100,000 p-yrs) was 3.4 times that among other enlisted personnel (106.0 per 100,000 p-yrs) and 5.6 times that among officers (64.7 per 100,000 p-yrs). Incident IM diagnoses peaked among recruits in 2007 (747.0 per 100,000 p-yrs) and 2009 (925.6 per 100,000 p-yrs) (data not shown).
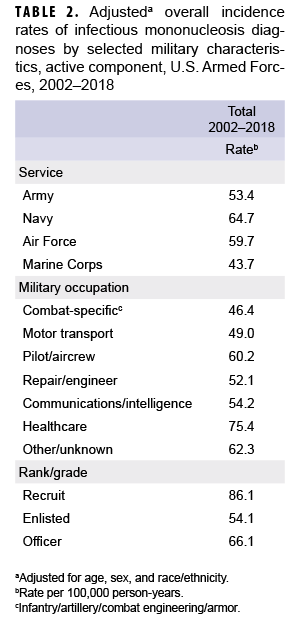
After adjusting for age, sex, and race/ethnicity, the incidence rate of IM diagnoses was highest among service members in the Navy (64.7 per 100,000 p-yrs) and lowest among those in the Marine Corps (43.7 per 100,000 p-yrs) (Table 2). Across military occupations, the adjusted overall rate was highest among health care workers (75.4 per 100,000 p-yrs) and lowest among those in combat-specific occupations. Finally, the adjusted overall rate of incident IM diagnoses among recruits was 86.1 per 100,000 p-yrs, which was 1.6 times that among other enlisted personnel and 1.3 times that of officers (Table 2).
By location
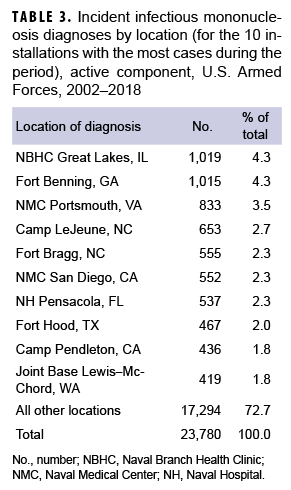
During the 17-year surveillance period, the medical treatment facilities at 10 installations diagnosed at least 400 incident cases of IM each; when combined, these installations diagnosed more than one-quarter (27.3%) of all cases (Table 3). Of these 10 installations, 2 provide support to recruit/basic combat training centers (Naval Branch Health Clinic [NBHC] Great Lakes, IL; Fort Benning, GA) and 4 support large combat troop populations (Camp Lejeune, NC; Fort Bragg, NC; Fort Hood, TX; Camp Pendleton, CA).
NBHC Great Lakes contributed the most incident cases of IM during the years 2002–2006, with the greatest number of cases documented in 2005 (n=246) (data not shown). In 2007 (n=188), 2009 (n=138), and 2010 (n=76), Fort Benning diagnosed the greatest number of incident cases. Joint Base Lewis–McChord contributed the greatest number of cases in 2008 (n=76). In 2011 (n=41), 2012 (n=80), and 2013 (n=80), Camp Lejeune had the greatest number of cases. Naval Medical Center Portsmouth recorded the most incident cases during the years 2014–2018, with the greatest number occurring in 2017 (n=148) (data not shown).
Burden
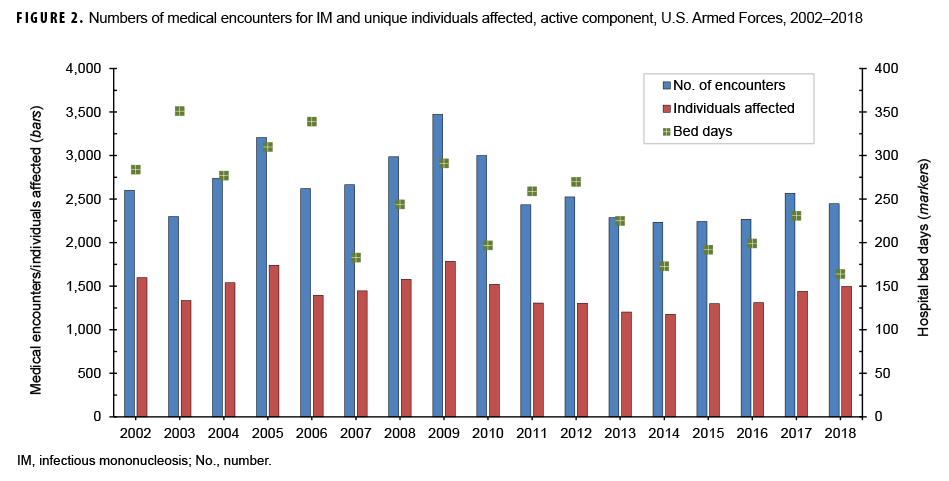
During the surveillance period, there were 44,606 total medical encounters and 4,189 hospital bed days for IM among 23,861 individuals (Figure 2). The annual number of encounters per person fluctuated between 1.6 and 2.0 throughout the 17-year period (mean=1.8). There were peaks in the total numbers of IM-related medical encounters in 2005 (3,206 encounters) and 2009 (3,474 encounters). A peak in the number of hospital bed days occurred in 2003, at 351 days.
Editorial Comment
This report demonstrates that the crude overall incidence rate of IM diagnoses among active component service members has remained stable over many years, at about 100 per 100,000 p-yrs. Compared to their respective counterparts, younger service members and, in particular, recruits had the highest overall rates of incident IM diagnoses as did females and non-Hispanic whites.
Using data and associated serum samples from the U.S. National Health and Nutrition Examination Survey from 2003 through 2010, Balfour and colleagues showed that the overall age-adjusted EBV antibody prevalence was estimated to be considerably higher among non-Hispanic blacks (88%) compared to non-Hispanic whites (64%).11 Similar results were reported from a study using data from children and adolescents who provided serum samples during the course of care at outpatient clinics in Minnesota. It is speculated that a combination of genetic susceptibility, family practices, and/or shared environment could play a role in acquisition of primary EBV infection before adolescence, which may account for this difference in antibody prevalence.11,17 This finding could also explain why the incidence of IM diagnoses in the current analysis was higher among non-Hispanic whites, as they are less likely to have been exposed to EBV before joining military service.
In the U.S. military, IM has the potential to reduce military operational readiness by contributing to lost or limited duty time. If each patient with an incident diagnosis of IM is unable to perform their duties for 2 weeks following infection, in an average year, cases of IM could result in an estimated 2,797 weeks of lost duty time each year. The recruit training environment likely contributes to increased risk of IM transmission because of close and crowded living conditions as well as physical and psychological stresses that could temporarily depress immune system function.18 Recruits who acquire IM during boot camp are at risk of being "recycled" —or having to repeat weeks of training with a new unit in an earlier cycle of training.
Currently, there is no vaccine available to prevent EBV infection; transmission can be avoided by not kissing infected individuals and by not sharing food, eating utensils, or drinking glasses with such individuals. Symptomatic treatment for IM consists mainly of supportive care and includes drinking fluids to stay hydrated, getting adequate rest and nutrition, and taking antipyretics and anti-inflammatory medications to reduce fever, throat discomfort, and malaise.1 In addition, there has been some evidence to suggest that vitamin D may reduce the risk of developing acute mononucleosis by helping to suppress viruses and by controlling the inflammatory response.19,20
A recent systematic review found insufficient evidence for the use of corticosteroid therapy for symptom control in IM and that data on long-term efficacy and side effects were limited.21 Corticosteroid therapy for routine cases of IM is not recommended because of concerns regarding immunosuppression during a clinical illness with a virus (EBV) that has been linked to a variety of malignancies (e.g., Burkitt's lymphoma, Hodgkin's lymphoma, and nasopharyngeal carcinoma).22,23 However, corticosteroid use is warranted in the management of IM patients with impending airway obstruction.24 Studies of the treatment of acute IM with the antiviral agents (i.e., acyclovir, valomaciclovir, and valacyclovir) have shown short-term suppression of oral viral shedding, but significant clinical benefit has not been demonstrated.25–28 Moreover, the use of these medications in the treatment of acute IM raises concerns regarding the potential for adverse events and antiviral resistance.28
Because more than 50% of patients with IM develop enlarged spleens within the first 2 weeks of symptom onset, a primary concern is avoiding activities that may lead to splenic rupture.29 Athletes with IM who are planning to resume contact sports are generally advised to gradually restart training 3 weeks after symptom onset. For strenuous contact sports or activities associated with increased intra-abdominal pressure (e.g., weightlifting), the general recommendation is to wait to resume such activities for a minimum of 4 weeks after symptom onset.30,31 A recent systematic review advocated individualized return-to-play recommendations for athletes with IM because of the variable disease course and lack of evidence-based guidelines.
There are several limitations that should be considered when interpreting the results of this analysis. The incidence of IM may be underestimated to the extent that affected individuals do not seek care and do not receive a diagnosis for IM, and asymptomatic cases are unlikely to be captured in this analysis. Alternatively, the incidence may be overestimated to the extent that a presumptive diagnosis was made without laboratory confirmation. Finally, the new electronic health record for the MHS, MHS GENESIS, was implemented at several military treatment facilities during 2017. Medical data from sites that are using MHS GENESIS are not available in the DMSS. These sites include Naval Hospital Oak Harbor, Naval Hospital Bremerton, Air Force Medical Services Fairchild, and Madigan Army Medical Center. Therefore, medical encounters for individuals seeking care at any of these facilities during 2017–2018 were not included in this analysis.
IM is not considered to be a serious illness; however, it can impact duty requirements during the acute phase. Military service members and recruit trainees in particular will continue to present with IM. Military commanders should be aware of the risks of infection as well as how to limit transmission within their units.
References
- Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/epstein-barr/aboutmono.html. Accessed 11 May 2019.
- Dunmire SK, Hogquist KA, Balfour HH. Infectious mononucleosis. Curr Top Microbiol Immunol. 2015;390:211–240.
- Hurt C, Tammaro D. Diagnostic evaluation of mononucleosis-like illnesses. Am J Med. 2007;120(10): e911e.1–911e.8.
- Fugl A, Andersen CL. Epstein-Barr virus and its association with disease–a review of relevance to general practice. BMC Fam Pract. 2019;20(1):62.
- Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med. 2000;109:531–537.
- Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575–580.
- Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124(1):189–193.
- White PD, Thomas JM, Amess J, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry. 1998;173:475–481.
- Vetsika EK, Callan M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev Mol Med. 2004;6(23):1–16.
- Fafi-Kremer S, Morand P, Brion JP, et al. Long-term shedding of infectious Epstein-Barr virus after infectious mononucleosis. J Infect Dis. 2005;191(6):985–989.
- Balfour HH Jr, Odumade OA, Schmeling DO, et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J Infect Dis. 2013;207(1):80–88.
- Lennon P, Crotty M, and Fenton JE. Infectious mononucleosis. BMJ. 2015;350:h1825.
- Williams-Harmon YJ, Jason LA, Katz BZ. Incidence of infectious mononucleosis in universities and U.S. military settings. J Diagn Tech Biomed Anal. 2016;5(1).
- Candy B, Chalder T, Cleare AJ, Wessely S, White PD, Hotopf M. Recovery from infectious mononucleosis: a case for more than symptomatic therapy? A systematic review. Br J Gen Pract. 2002;52(483):844-851.
- Army Medical Surveillance Activity. Infectious mononucleosis among active duty US service members, 1998–1999. MSMR. 2001;7(2):2–8.
- Armed Forces Health Surveillance Branch. Absolute and relative morbidity burdens attributable to various illnesses and injuries, active component, U.S. Armed Forces, 2018. MSMR. 2019;26(5):2–10.
- Condon LM, Cederburg LE, Rabinovitch MD, et al. Age-specific prevalence of Epstein-Barr virus infection among Minnesota children: effects of race/ethnicity and family environment. Clin Infect Dis. 2014;59(4):501–508.
- Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103(2):693–699.
- Maloney SR, Almarines D, Goolkasian P. Vitamin D levels and monospot tests in military personnel with acute pharyngitis: a retrospective chart review. PLoS One. 2014;9(7):e101180.
- Maghzi H, Ataei B, Khorvash F, Yaran M, Maghzi A. Association between acute infectious mononucleosis and vitamin D deficiency. Viral Immunol. 2016;29(7):398–400. Rezk E, Nofal YH, Hamzeh A, Aboujaib MF, AlKheder MA, Al Hammad MF. Steroids for symptom control in infectious mononucleosis. Cochrane Database Syst Rev. 2015;(11):CD004402.
- Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349(14):1324–1332.
- Cohen JI. Epstein-Barr virus vaccines [published correction appears in Clin Transl Immunol. 2015;4(4)e36]. Clin Transl Immunol. 2015;4(1):e32.
- Kakani S. Airway compromise in infectious mononucleosis: a case report. Cases J. 2009;2:6736.
- van der Horst C, Joncas J, Ahronheim G, et al. Lack of effect of peroral acyclovir for the treatment of acute infectious mononucleosis. J Infect Dis. 1991;164(4):788–792.
- Tynell E, Aurelius E, Brandell A, et al. Acyclovir and prednisolone treatment of acute infectious mononucleosis: a multicenter, double-blind, placebo-controlled study. J Infect Dis. 1996;174(2):324–331.
- Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis. 1999;31(6):543–547.
- De Paor M, O’Brien K, Fahey T, Smith SM. Antiviral agents for infectious mononucleosis (glandular fever). Cochrane Database Syst Rev. 2016;12:CD011487.
- Hosey RG, Kriss V, Uhl TL, DiFiori J, Hecht S, Wen DY. Ultrasonographic evaluation of splenic enlargement in athletes with acute infectious mononucleosis. Br J Sports Med. 2008;42(12):974–977.
- Auwaerter PG. Infectious mononucleosis: return to play. Clin Sports Med. 2004;23(3):485–487.
- Becker JA, Smith JA. Return to play after infectious mononucleosis. Sports Health. 2014;6(3):232–238.