What Are the New Findings?
This is the first report of the effect of infectious diarrhea on British troops participating in the Askari Storm Training Exercise in Nanyuki, Kenya. These data highlight the impact of diarrheal diseases in this training exercise and the continued need to refine primary prevention strategies.
What Is the Impact on Readiness and Force Health Protection?
In 2019, the National Center for Medical Intelligence identified bacterial diarrheal disease as the number one priority among endemic and emerging infectious diseases that pose a threat to deployed U.S. forces. These data support the importance of diarrheal disease in training exercises in addition to operational settings.
Abstract
Travelers’ diarrhea (TD) has historically been common among deployed military personnel and remains a leading infectious disease threat to this population. The risk factors, work performance, and illness associated with TD among British active duty service members exercising at British Army Training Unit Kenya (BATUK) were assessed. Members of the British Army who were finishing a 6-week combined arms training exercise in Nanyuki, Kenya, completed routine public health surveillance questionnaires. Survey data included information on demographics, rank, risk factors, illness characteristics, and impact on performance. Among 1,227 survey respondents, 21.9% (n=269) reported having diarrhea, with an estimated 824 days of total missed work and 1,215 days of work under-performance. The majority of cases (54.6%) had multiple diarrhea episodes. One quarter (24.9%) of the respondents with TD sought medical care and 19.7% were bedded down because of their illness. There were no statistically significant differences between the TD and no TD groups on the demographic characteristics examined. The strongest risk factor for diarrhea was having a colleague with diarrhea (adjusted odds ratio=51.78; 95% confidence interval: 29.44–91.06). TD had a notable impact on duty status and operational capability. Efforts are needed to improve BATUK's participant education on the importance of diarrheal disease prevention and management.
Background
Travelers' diarrhea (TD) is typically defined as 3 or more unformed stools in 24 hours with at least 1 of the following additional symptoms: fever, nausea, vomiting, abdominal cramps, tenesmus, or bloody stools.1 TD has been the most common medical problem and remains one of the most important health threats among globally deployed military members, both in combat and peacetime missions.2,3 Infectious gastroenteritis was identified as 1 of the top 5 reasons for clinic visits in studies evaluating disease and non-battle injury rates in recent peacetime and combat operational settings.4 While TD typically spontaneously resolves within 3 to 5 days,5 it can have detrimental long-term consequences including functional bowel disorders, reactive arthritis, and Guillain-Barré syndrome.6,7
The most recent systematic review of TD in military (and similar) populations estimated more than 35 cases per 100 person-months with significant heterogeneity in incidence depending on region and duration of travel.5 Studies of the etiology of TD in military populations point to the importance of diarrheagenic Escherichia coli, in particular enterotoxigenic E. coli, as well as other bacterial etiologies as key pathogens. TD significantly impacts affected individuals with up to 1 in 5 personnel being incapacitated or being placed sick in quarters (SIQ)5 and a high proportion of those affected experiencing prolonged decreases in job performance.8
In addition to high rates of TD in operational settings, prior studies have highlighted elevated rates during military training exercises.9–12 Data for the current study were obtained from members of the British Army who completed a 6-week combined arms training exercise based at British Army Training Unit Kenya (BATUK), a permanent training support unit located 200 kilometers north of Nairobi in Nanyuki, Kenya. BATUK consists of approximately 200 permanent and short-tour staff. BATUK provides logistical support to visiting units and, through an agreement with the Kenyan Government, facilitates six-week exercises for up to 6 infantry battle groups (1,000–1,200 troops) per year.9 In addition, BATUK hosts Royal Engineer Squadron exercises that carry out civil engineering projects and a medical company group deployment that provides outreach primary health care assistance to the civilian community in collaboration and coordination with the regional and local Kenyan Health authorities.
Methods
Study design
This was a cross-sectional study of TD among UK Army Infantry Battle Groups participating in training exercises at BATUK training camp between Jan. and June 2014. Data were collected as part of routine public health surveillance from 1 of 3 battle groups of military personnel participating in the Askari Storm training exercise. These 6-week exercises involved a light infantry battle group operating over the rugged terrain of the Kenya savannah, including river crossings and water exposure at an altitude of 800–1,500 meters. Soldiers largely lived in the field in bivouacs and returned to camp accommodation 2–3 times during an exercise. Food and water on exercise were provided from ration packs or prepared by military chefs, with oversight from the military environmental health team. There were typically opportunities at the end an exercise for individuals and groups to take part in outdoor training activities that included kayaking and other water-based activities. During such activities, food was provided from Kenyan sources and there was fresh water exposure. During the training exercise, soldiers had opportunities to eat at Kenyan restaurants local to the base. These restaurants were inspected by the military environmental health team and required to meet UK food hygiene standards in order to appear on the approved list; however, enforcement of these regulations sometimes proved challenging.
Participants completed a survey consisting of 28 questions that collected information on respondent demographic characteristics, illness characteristics, impact of illness on work performance, and potential risk factors for TD. The questionnaires were designed for ease of use and simplicity of understanding. To facilitate data analysis, the questionnaire allowed few options for free text entries. To obtain information on illness characteristics, respondents were asked to indicate the number of different diarrheal episodes experienced and to describe the number of bowel movements from their self-assessed worst diarrheal episode. To determine the impact of TD on work performance, respondents were asked about their ability to perform their normal duties during an average episode of diarrhea and the total number of days of work missed as a result of TD. No personally identifiable information was collected at any point during the surveillance exercise.
Data sources
All UK Army Infantry Battle Group personnel participating in the 6-week Askari Storm training exercise were asked to complete routine self-administered public health surveillance paper questionnaires. Respondents were members of 1 of 3 separate battle groups (selected at random) of approximately 1,000 personnel each. Questionnaires were distributed by BATUK staff and completion was voluntary and anonymous. No data were obtained on personnel who did not complete the questionnaire. Survey data were entered into a Microsoft Access database (2010, Microsoft Corporation, Redmond, WA) by a trained data manager. Institutional review board approval was not required as per the Ministry of Defence Research Ethics Committee guidelines.
Statistical analysis
Self-reported data on demographic characteristics, potential risk factors, and diarrhea illness characteristics were summarized. Demographic comparisons between those with and without diarrhea were made using a Pearson’s chi-square test. Separate logistic regression models were used to calculate odds ratios (ORs) and associated 95% confidence intervals (CIs) for each potential TD risk factor. For those potential risk factors with statistically significant ORs, adjusted odds ratios (AORs) were calculated from a multivariable logistic regression model that included all potential risk factors. All statistical analyses were performed using SAS/STAT software, version 9.3 (2011, SAS Institute, Cary, NC).
Results
Study population
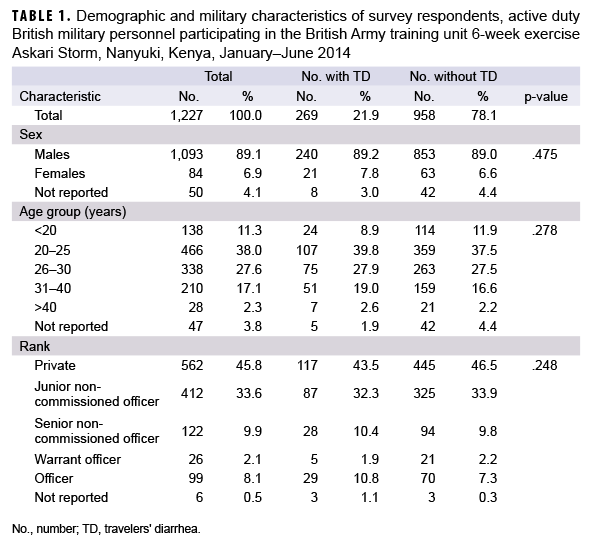
A total of 1,227 British Army military personnel who participated in the training exercise at the BATUK Askari Storm training camp completed public health surveillance questionnaires (Table 1). Given that each battle group had approximately 1,000 military members, an approximate estimate of the size of the group from which the sample population was drawn was 3,000, for an estimated response rate of about 41%. Survey respondents were predominantly male (89.1%), between the ages of 20–25 years old (38.0%) and of the "Private" rank (45.8%). A total of 21.9% of the respondents reported having diarrhea; there were no statistically significant differences between the TD and no TD groups on the demographic characteristics examined (Table 1).
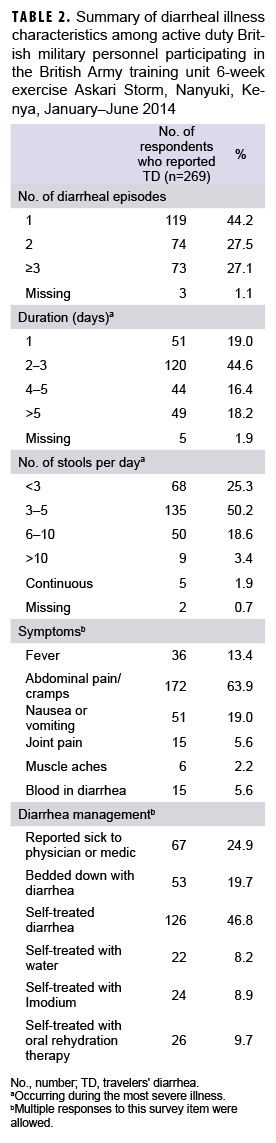
The majority of respondents with diarrhea (n=147; 54.6%) reported having multiple individual diarrheal episodes, and more than one-third (34.6%) of affected respondents had a diarrheal illness that lasted for more than 3 days (Table 2), leading to a total of 824 days of missed work (data not shown). During their worst diarrheal episode, 50.2% of TD cases reported 3–5 stools a day, with almost a quarter (23.9%) reporting more than 5 stools daily. Concurrent symptoms included abdominal pain or cramps (63.9%), nausea or vomiting (19.0%), subjective fever (13.4%), joint pain (5.6%), and muscle aches (2.2%). Only a quarter (n=67; 24.9%) of the respondents with TD sought care and 19.7% were "bedded down" because of their illness (Table 2).
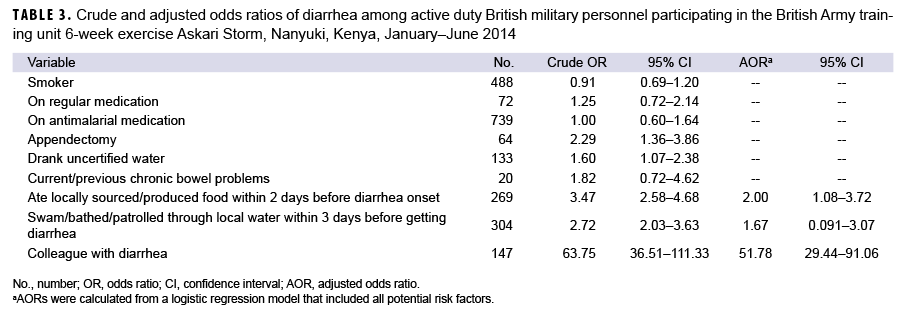
Results of bivariate analyses suggested that significant risk factors for TD included having a colleague with diarrhea (OR=63.75; 95% CI: 36.51–111.33), consumption of locally sourced/produced food within 2 days before diarrhea onset (OR=3.47; 95% CI: 2.58–4.68), swimming/bathing/patrolling through local water within 3 days before diarrhea onset (OR=2.72; 95% CI: 2.03–3.63), prior appendectomy (OR=2.29; 95% CI: 1.36–3.86), and consumption of uncertified water (OR=1.60; 95% CI: 1.07–2.38) (Table 3). In a multivariable model including all potential risk factors, only the consumption of locally sourced/produced food (AOR=2.00; 95% CI: 1.08–3.72) and having a colleague with diarrhea (AOR=51.78; 95% CI: 29.44–91.06) remained statistically significant.
Editorial Comment
Accurate estimates of the burden of disease in different geographical settings and types of activities assist with military planning. The results of this study highlight the continued importance of TD in this population. In this self-selected sample of a military population engaged in a training exercise, TD increased the number of duty days lost and subsequently reduced the full benefit of the training exercise. The proportion of respondents who reported having diarrhea is comparable to estimates in previously published reviews5;however, the high proportion who reported repeated diarrheal episodes over the short duration of the exercise is notable. Additionally, these data enable power calculations for planned interventional trials assessing diarrhea prevention tools in these settings. These data also suggest that the development of the training site and control of the environment and training population may have reduced the proportion reporting TD in this cycling population since 1999–2000 when the proportion with diarrhea was estimated at 40–60% (UK Ministry of Defense, unpublished internal report, Feb. 2001). Risk factors similar to those in prior studies were identified11,13–15; however, by far the greatest risk factor was having a colleague with TD. This may represent a common point-source exposure or person-to-person spread. Based on the typical etiology of TD, and the transmission of common pathogens, the former is more likely than the latter.
Participants were unlikely to seek treatment for TD, consistent with other studies on care-seeking behavior in cases of TD.16 This may be due to the illness being less severe, perception of a lack of viable treatment strategies, and/or social constraints resulting in decreased reporting. Self-treatment was relatively rare; however, 8.9% of participants with diarrhea used the antimotility agent, loperamide. Recent studies have shown that treatment regimens that include an antimotility agent and an appropriate antibiotic can produce rapid clinical resolution and minimize lost duty time.17
There are several limitations that should be considered when interpreting the results of this study. First, the reliance on self-reported data may have introduced response bias. It is possible that those who had at least 1 diarrheal episode may have been more likely to complete the questionnaire than those who did not. It is also possible that questionnaire respondents misclassified their exposure to potential risk factors, the outcome of diarrhea, and/or the characteristics of their illnesses. In addition, participants may have mischaracterized concomitant symptoms that may not have occurred concurrent with their diarrheal illnesses. Prior studies have also highlighted underreporting of TD in deployment settings.18 Moreover, data on the background characteristics of soldiers who were eligible to complete the survey but who did not do so would have helped to assess the generalizability of the results to the larger training population. Finally, efforts to validate the survey or to assess its internal validity and reliability were limited, which may have affected the results.
Ongoing data collection is needed to assess the continued disease risk associated with TD and other potential infectious disease threats of concern. Additionally, efforts to understand the pathogens causing disease in this population are needed to ensure appropriately targeted interventions and treatments. Utilization of recently established clinical practice guidelines for TD treatment, while not preventing TD, may minimize the impact of the illness on personnel.19
Author affiliations: Military Enteric Disease Group, Academic Department of Military Medicine, Royal Centre for Defence Medicine, Birmingham, UK (Maj Burns and Dr. Connor); Enteric Disease Department, Infectious Disease Directorate, Naval Medical Research Center, Silver Spring, MD (Dr. Porter, CAPT Gutierrez, Ms. McDavid, and Dr. Riddle); Centre of Defence Pathology, RCDM, Birmingham, UK (Lt Col Hutley).
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government nor that of the UK Ministry of Defence. Authors are military service members (or employees of the U.S. Government). This work was prepared as part of official duties. Title 17 U.S.C. §105 provides that "Copyright protection under this title is not available for any work of the United States Government." Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. Disclosure: This study was exempt from the Ministry of Defence Research and Ethics Committee approval (MOD JSP 536 dated 21 Jan. 2011) and was conducted under support of the Military Infectious Disease Research Program.
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hill DR, Beeching NJ. Travelers' diarrhea. Curr Opin Infect Dis. 2010;23(5):481–487.
- Connor P, Porter CK, Swierczewski B, Riddle MS. Diarrhoea during military deployment: current concepts and future directions. Curr Opin Infect Dis. 2012;25(5):546–554.
- Sanders JW, Putnam SD, Riddle MS, Tribble DR. Military importance of diarrhea: lessons from the Middle East. Curr Opin Gastroenterol. 2005;21(1):9–14.
- Letizia A, Riddle MS, Tribble D, et al. Effects of pre-deployment loperamide provision on use and travelers' diarrhea outcomes among U.S. military personnel deployed to Turkey. Travel Med Infect Dis. 2014;12(4):360–363.
- Olson S, Hall A, Riddle MS, Porter CK. Travelers' diarrhea: update on the incidence, etiology and risk in military and similar populations—1990–2005 versus 2005–2015, does a decade make a difference? Trop Dis Travel Med Vaccines. 2019;5:1.
- Porter CK, Thura N, Riddle MS. Quantifying the Incidence and burden of postinfectious enteric sequelae. Mil Med. 2013;178(4):452–469.
- Pogreba-Brown K, Austhof E, Armstrong A, et al. Chronic gastrointestinal and joint-related sequelae associated with common foodborne illnesses: A scoping review. Foodborne Pathog Dis. 2020;17(2):67–86.
- Putnam SD, Sanders JW, Frenck RW, et al. Self-reported description of diarrhea among military populations in operations Iraqi Freedom and Enduring Freedom. J Travel Med. 2006;13(2):92–99.
- Sanders JW, Isenbarger DW, Walz SE, et al. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: Presentation and outcome of Campylobacter infection. Am J Trop Med Hyg. 2002;67(5):533–538.
- Tribble DR, Baqar S, Pang LW, et al. Diagnostic approach to acute diarrheal illness in a military population on training exercises in Thailand, a region of campylobacter hyperendemicity. J Clin Microbiol. 2008;46(4):1418–1425.
- Sebeny PJ, Nakhla I, Moustafa M, et al. Hotel clinic-based diarrheal and respiratory disease surveillance in U.S. service members participating in Operation Bright Star in Egypt, 2009. Am J Trop Med Hyg. 2012;87(2):312–318.
- Kasper MR, Lescano AG, Lucas C, et al. Diarrhea outbreak during U.S. military training in El Salvador. PLoS One. 2012;7(7):e40404.
- Porter CK, Riddle MS, Tribble DR, et al. The epidemiology of travelers' diarrhea in Incirlik, Turkey: a region with a predominance of heat-stabile toxin producing enterotoxigenic Escherichia coli. Diagn Microbiol Infect Dis. 2010;66(3):241–247.
- Hameed JM, McCaffrey RL, McCoy A, et al. Incidence, etiology and risk factors for travelers' diarrhea during a hospital ship-based military humanitarian mission: Continuing Promise 2011. PLoS One. 2016;11(5):e0154830.
- Lalani T, Maguire JD, Grant EM, et al. Epidemiology and self-treatment of travelers' diarrhea in a large, prospective cohort of department of defense beneficiaries. J Travel Med. 2015;22(3):152–160.
- Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (U.S. military and similar populations): A systematic review. Am J Trop Med Hyg. 2006;74(5):891–900.
- Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler's diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(8):1007–1014.
- Riddle MS, Tribble DR, Putnam SD, et al. Past trends and current status of self-reported incidence and impact of disease and nonbattle injury in military operations in Southwest Asia and the Middle East. Am J Public Health. 2008;98(12):2199–2206.
- Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers' diarrhea: a graded expert panel report. J Travel Med. 2017;24(suppl_1):S57–S74.