What Are the New findings?
This study describes the characteristics of COVID-19 cases among U.S. service members to date, including the prevalence of pre-existing comorbidities, vaccination status, and the proportion of cases hospitalized. This report also describes the characteristics of service members who died from COVID-19 during the surveillance period.
What Is the Impact on Readiness and Force Health Protection?
Detailed data on demographic characteristics, underlying medical conditions, and vaccination status can inform targeted communication to encourage persons in at-risk groups to practice preventive measures and promptly seek medical care if they become ill. Enhanced surveillance efforts can enable actions to prevent and control the current COVID-19 epidemic, which threatens the health of the force.
Abstract
Between Jan. 1, 2020 and Aug. 31, 2021, a total of 189,239 service members were identified as confirmed or probable cases of COVID-19. The majority of cases were male (81.2%) and 57.3% were aged 20–29 years. Overall, 19.2% had a diagnosis of at least 1 of the pre-existing comorbidities of interest in the year prior to becoming a case. The most common pre-existing comorbidity was obesity or overweight (5.2%), followed by cardiovascular disease (4.2%), and substance use disorder including nicotine dependence (4.0%). Service members who were hospitalized for COVID-19 were twice as likely to have a diagnosis of any pre-existing comorbidity compared to those who were not hospitalized. There were a total of 1,760 hospitalizations (0.9%) and 45 deaths reported among service members. In addition, there were 11,899 cases observed among fully vaccinated individuals; however, only 0.4% of hospitalized cases were fully vaccinated and no service member deaths occurred among fully vaccinated individuals during the surveillance period, highlighting the importance of COVID-19 vaccination for force health protection.
Background
Since the beginning of the COVID-19 pandemic, there have been over 249 million COVID-19 cases and 5 million COVID-related deaths worldwide, including over 46 million cases and over 754 thousand deaths in the U.S.1 Although most symptomatic cases of COVID-19 are mild, severe disease occurs in any demographic group.2,3 Older individuals and those with underlying medical conditions are at higher risk of serious illness and death; this risk increases with advancing age.3 The majority of COVID-19 deaths occur among adults aged 60 or older and among persons with serious underlying health conditions such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions (e.g., heart failure, coronary artery disease, cardiomyopathies), an immunocompromised state from solid organ transplant, and obesity (body mass index [BMI]=30–39.9 kg/m2).4,5
Two mRNA vaccines for COVID-19 (Moderna and Pfizer-BioNTech) were approved in December 2020 and an adenovirus-vectored vaccine (Janssen) was approved in late February 2021. Randomized clinical trials have shown that the current mRNA-based COVID-19 vaccines have high efficacies (94%–95%) for preventing COVID-19.6,7 The Janssen adenovirus vectored vaccine also had moderately high efficacy against symptomatic COVID-19 (66%) and high efficacy against hospitalization for COVID-19 (93%).8,9 Since these vaccines became available and used in the U.S., they have had substantial impacts on mitigating COVID-19 outbreaks and reducing risk of hospitalization and death.10 Concerns were initially raised about the effectiveness of these vaccines against emerging new strains of SARS-CoV-2, including the Delta (B.1.617.2) variant, which became the predominant variant of the virus in the U.S. in July 2021.12 There is currently very limited information about real-world effectiveness of the Janssen vaccine against the Delta variant. However, several studies have indicated moderate to high effectiveness of the Moderna and Pfizer-BioNTech vaccines against the Delta variant, particularly for preventing hospitalization and death.12–14
The Department of Defense (DOD) began COVID-19 vaccination in mid-December 2020. These vaccines were originally made available to service members on a voluntary basis according to occupational risk, but were made mandatory on 24 August 2021.15 The objective of this study was to provide an update on the numbers of probable and confirmed COVID-19 cases among U.S. military service members in addition to describing demographics, prevalence of pre-existing comorbidities, hospitalization rates, and deaths.
Methods
Since March 2020, the Armed Forces Health Surveillance Division (AFHSD) has maintained a case list of MHS beneficiaries with COVID-19. This list is updated daily and comprises Composite Health Care System (CHCS) Health Level 7 (HL7)-formatted and MHS Genesis laboratory positive test results extracted by the Navy and Marine Corps Public Health Center Epi Data Center (for all services), as well as medical event reports of laboratory confirmed and probable COVID-19 infection derived from the Disease Reporting System Internet (DRSi). AFHSD also maintains the Defense Medical Surveillance System (DMSS), a continuously expanding relational database of personnel and medical data.
For this analysis, cases of COVID-19 among active and reserve/guard component service members were included if the incident date occurred within 90 days of a personnel demographic record maintained in the DMSS. Beneficiary status (i.e., active or reserve/guard component) and branch of military service were determined based on the information recorded in the DMSS. The incident date for each COVID-19 case was defined as the earliest date of onset recorded in the DRSi, or the earliest collection date for the sample that tested positive via PCR or antigen laboratory testing.
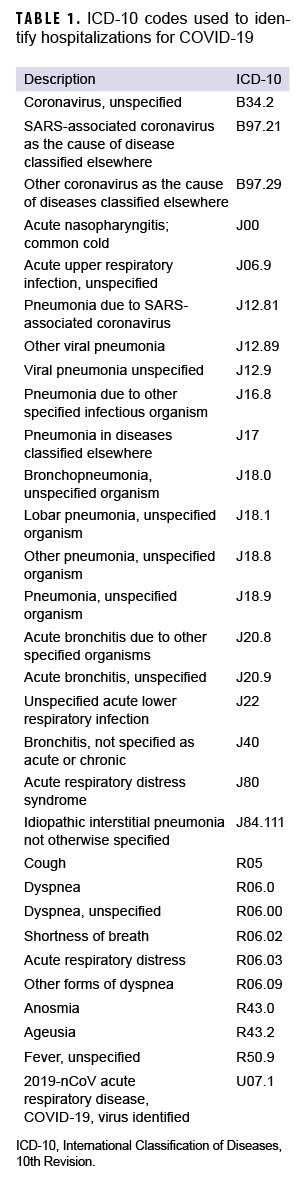
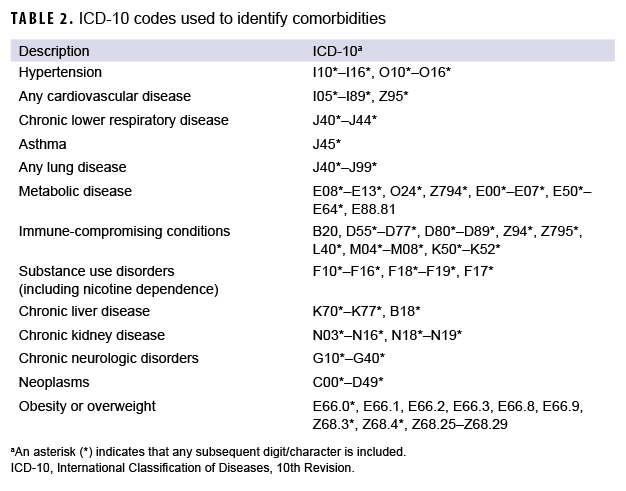
In addition, DMSS data were used to identify hospitalizations for COVID-19 and for demographic information that was missing from the case list. DMSS data were also used to identify comorbidities from administrative records of inpatient and outpatient medical encounters, which included encounters from fixed military treatment facilities as well as outsourced care reimbursed by TRICARE. A hospitalization for COVID-19 was defined by having an inpatient record in DMSS occurring within 30 days after the first case record for COVID-19, with the International Classification of Diseases, 10th Revision (ICD-10) code U07.1 in the first or second diagnostic position, or a diagnosis of COVID-like illness in the first or second diagnostic position (Table 1). An individual was considered to have a pre-existing comorbidity if there was an inpatient or outpatient encounter record containing an ICD-10 code for a comorbidity of interest within 365 days prior to the COVID-19 incident date (Table 2).
Data on vaccination status at the time of the incident COVID-19 infection were derived from immunization records in the DMSS. An individual was considered fully vaccinated at the time of the case if the incident date occurred at least 14 days after the second dose of the Moderna (vaccine administered [CVX] code=207) or Pfizer-BioNTech (CVX code=208) vaccine, or at least 14 days after the first dose of the Janssen vaccine (CVX code=212). An individual was considered partially vaccinated if the individual had received at least 1 dose of vaccine but did not meet the aforementioned 14-day criteria for full vaccination.
COVID-19-related deaths occurring among service members were tracked and reported separately from cases of COVID-19. These deaths were identified via communication with the Armed Forces Medical Examiner System and via media reports, Director's Critical Information Requirement reports, the DRSi, and chart review of service members' electronic health record in the Armed Forces Health Longitudinal Technology Application (AHLTA). Information documented in these sources were used to describe pre-existing comorbidities and clinical course among those who died from COVID-19.
Results
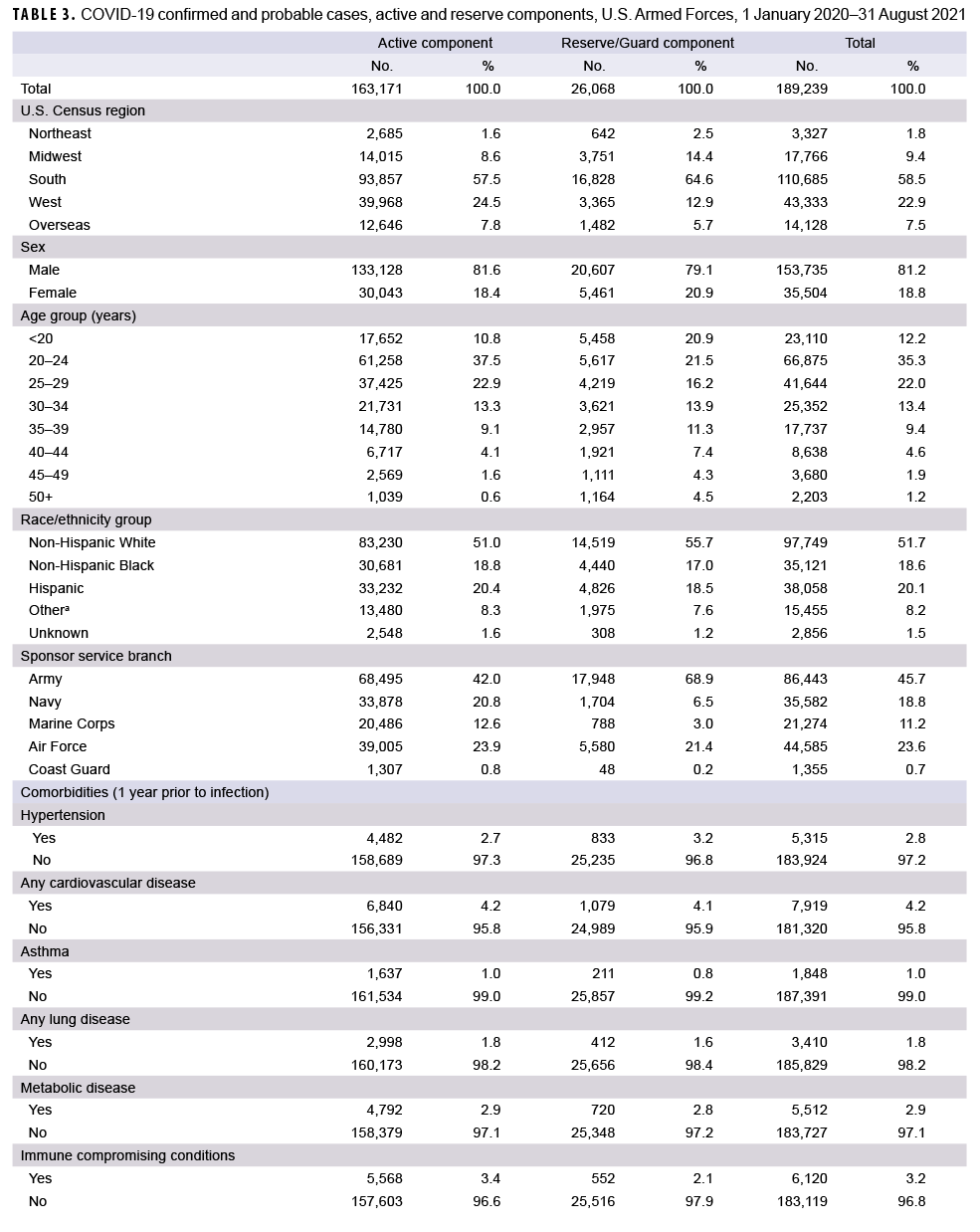
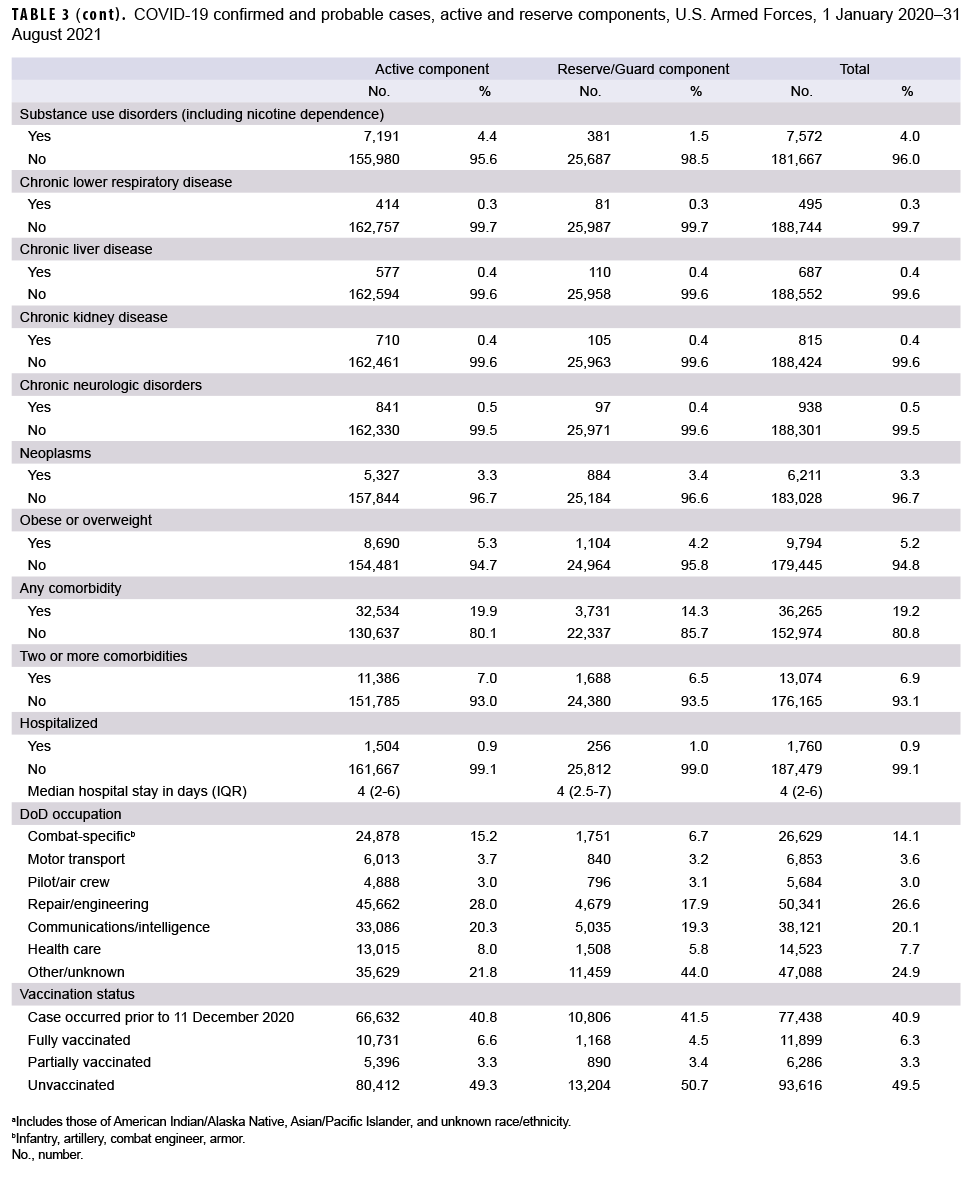
As of Aug. 31, 2021, a total of 189,239 service members were identified as confirmed or probable cases of COVID-19 (Table 3). Of these cases, 19.2% had a medical encounter for at least 1 of the pre-existing comorbidities of interest in the year prior to becoming a case, and 6.9% had an encounter for 2 or more pre-existing comorbidities. There were a total of 1,760 hospitalizations (0.9%) and 45 deaths. Of the 42 deaths with past medical history data available for review, 17 (40.5%) had 1 pre-existing comorbidity of interest, 15 (35.7%) had 2 or more comorbidities and 10 (23.8%) had no comorbidities. A total of 111,801 cases occurred after COVID-19 vaccinations became available to the DOD on 11 December 2020. Among these cases, 83.7% (n=93,616) occurred among unvaccinated service members, 10.6% (n=11,899) cases occurred among fully vaccinated individuals, and 5.6% (n=6,286) occurred among partially vaccinated individuals. A total of 48 hospitalizations for COVID-19 occurred among fully vaccinated service members and there were 44 hospitalizations among partially vaccinated service members (data not shown). No service member deaths occurred among vaccinated individuals, except for 1 active duty member who had received 1 dose of Pfizer vaccine 18 days prior to his death.
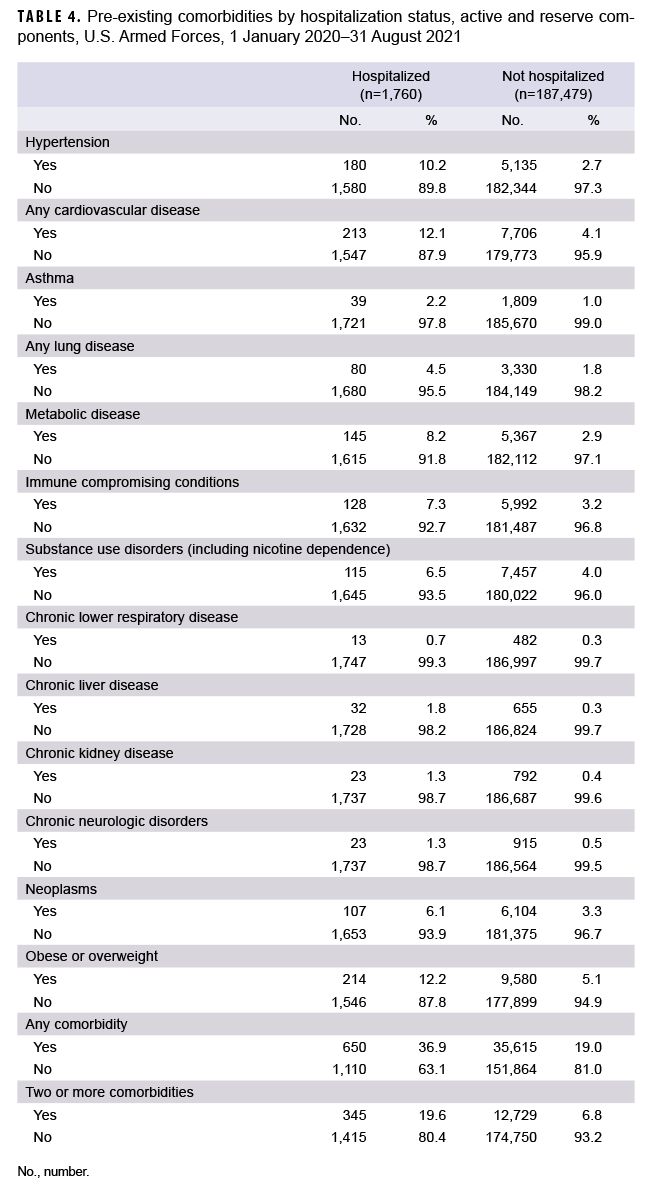
Service members who were hospitalized for COVID-19 were twice as likely to have a diagnosis of any comorbidity in the year prior to becoming a case compared to those who were not hospitalized, and almost 3 times as likely to have a diagnosis of multiple comorbidities (Table 4). Of note, those who were hospitalized were 5 times as likely to have a prior diagnosis of chronic liver disease and almost 4 times as likely to have a prior hypertension diagnosis, compared to those who were not hospitalized. Of the 32 service member deaths with hospitalization data available, 24 (75.0%) were hospitalized at the time of death, 8 (25.0%) were found unresponsive at home or at an alternate site, and 1 died en route to the hospital.
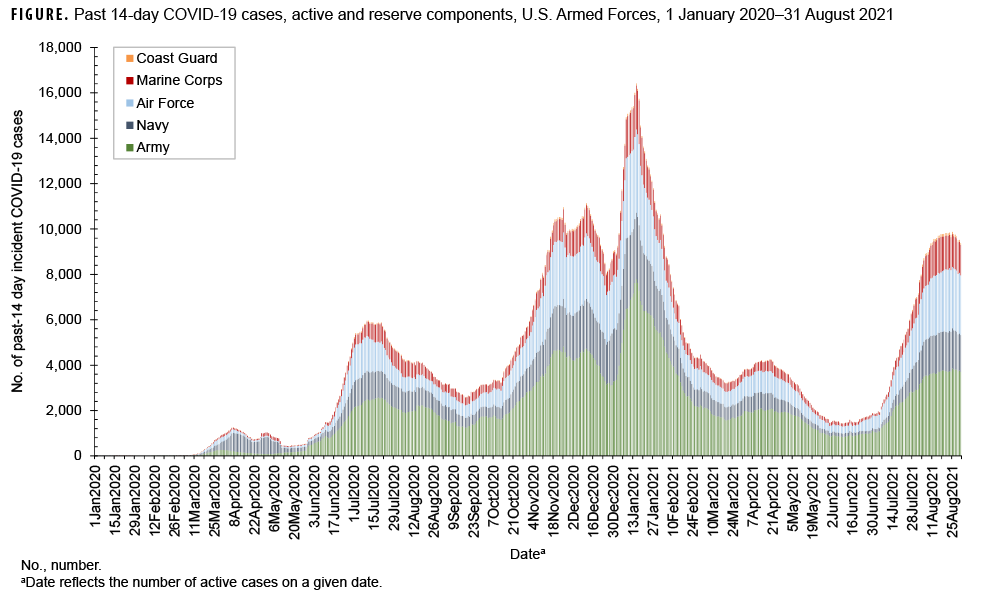
The highest peak in the number of past 14-day incident COVID-19 cases among all service members occurred on Jan. 15, 2021 with a total of 16,418 past 14-day cases, and was preceded by a peak on Dec. 11, 2020 of 11,131 past 14-day cases (Figure). Following the peak on Jan. 15, 2021, there was another peak on Aug. 25, 2021 of 9,875 past 14-day cases coinciding with the period of transmission of the Delta variant.
Active component
A total of 163,171 active component members had been infected with the SARS-CoV-2 virus as of 31 August 2021 (Table 3). The largest demographic proportions of cases were among those who were male (81.6%), aged 20–24 (37.5%), non-Hispanic White (51.0%), and in the Army (42.0%). About one-fifth (19.9%) of the infected cases had a diagnosis of a pre-existing comorbidity in the year prior to becoming a case, and 7.0% were diagnosed with 2 or more comorbidities. Of the comorbidities that were assessed, the most commonly diagnosed were obesity or overweight (n= 8,690; 5.3%), substance use including nicotine dependence (n= 7,191; 4.4%), and cardiovascular disease (n= 6,840; 4.2%). A total of 1,504 active component members (0.9%) were hospitalized, with a median hospital stay of 4 days (interquartile range [IQR]=2–6). A total of 10,731 cases (11.1%) among the 96,539 cases that occurred after Dec. 11, 2020 were fully vaccinated, and 5.6% (n=5,396) were partially vaccinated.
During the surveillance period, there were 17 deaths among active component COVID-19 cases (data not shown). Past medical history was available in AHLTA for all of the active component service member deaths reviewed. COVID-specific case-related information, including clinical progression of illness, was available for review in 12 (70.6%) AHLTA records and 17 (100.0%) corroborative clinical reports and summaries, typically prepared by the services following a death. Of the active component deaths, 7 (41%) were Army, 7 (41%) were Navy, 2 (12%) were Air Force, and 1 (6%) was Marine Corps. The average age at death was 39 years. A majority (82.4%; n=14) of those who died had at least 1 comorbidity of interest and 52.9% (n=9) had a BMI that placed them in the overweight or obese category. The 2 most common pre-existing comorbidities among those who died were obesity (52.9%; n=9) and cardiovascular disease, primarily hyperlipidemia (23.5%, n=4). At the time of death, 11 service members were hospitalized, 4 were "found unresponsive" at home or at an alternative site and later pronounced dead in the emergency room, and 1 died en route to the hospital. Hospitalization data for 1 service member was not available. The clinical progression of illness for most hospitalized cases followed a standard pattern of deterioration: onset of symptomatic illness, increasing dyspnea (shortness of breath) with development of COVID pneumonia, ICU admission; onset of acute respiratory distress syndrome (ARDS) followed by intubation and mechanical ventilation, development of COVID-related complications (e.g., cardiogenic shock, myocarditis, deep vein thrombosis, disseminated intravascular coagulation, etc.) and death. None of the active component service members who died were fully vaccinated.
Reserve/Guard component
A total of 26,068 COVID-19 cases were identified among reserve and guard service members (Table 3). The largest proportions of cases were male (79.1%), aged 20–24 (21.5%), non-Hispanic White (55.7%), and in the Army (68.9%). About one-seventh (14.3%) of cases were diagnosed with a pre-existing comorbidity, and 6.5% were diagnosed with 2 or more comorbidities. Of the pre-existing comorbidities that were assessed, the most commonly diagnosed comorbidities were obesity or overweight (n=1,104; 4.2%), cardiovascular disease (n=1,079; 4.1%), and neoplasms (n=884; 3.4%). A total of 256 reserve/guard members (1.0%) were hospitalized, with a median hospital stay of 4 days (IQR=2.5–7). A total of 1,168 cases (7.7%) among the 15,262 cases that occurred after Dec. 11, 2020 were fully vaccinated, and 5.8% (n=890) were partially vaccinated.
There were 28 deaths among reserve and guard COVID-19 cases; of these, 8 were on active duty status at the time of death (data not shown). Past medical history was available in AHLTA for 25 (89.3%) of the reserve/guard deaths reviewed. COVID-specific case-related information, including clinical progression of illness, was available in AHLTA and corroborative clinical reports/summaries for all of the reserve/guard members who died while on active duty, but only available for 5 (25%) reserve/guard members who died while not on active duty. Similarly, vaccination status was available for all of the activated reserve/guard members who died, but only available for 11 (55%) of the inactivated reserve/guard members who died. Of the total reserve guard deaths, 19 (67.9%) were Army, 5 (17.9%) were Navy, and 4 (14.3%) were Air Force. The average age at death was 47 years. Of those with available data in AHLTA or in other sources, 15 (62.2%; 15/23) had at least 1 pre-existing comorbidity and 12 (54.5%; 12/22) had BMIs that placed them in the category of overweight or obese. Similar to the active component, the most common comorbidities among reserve/guard deaths were obesity (54.5%) and cardiovascular disease (25.0%; 6/24), with 4 having a diagnosis of hyperlipidemia. Of the 11 inactive reserve/guard service members with available COVID-specific case-related data, 8 (72.7%) were hospitalized at the time of death with a clinical progression of illness that followed the standard pattern outlined above and 3 (27.3%) were found unresponsive at home or at an alternate site and later pronounced dead in the emergency room. None of the reserve/guard service members who died were fully vaccinated.
Editorial Comment
This report describes the demographic characteristics and prevalence of pre-existing comorbidities among service members with incident COVID-19 infection. Not surprisingly, cases among service members (including recruits) were most commonly diagnosed in young, non-Hispanic White males, which follows the expected demographic distributions of these groups. Overall, 19.2% had a medical encounter for at least 1 of the pre-existing comorbidities of interest in the year prior to becoming a case. The most common pre-existing comorbidities in both hospitalized and non-hospitalized cases were obesity or overweight, followed by cardiovascular disease. These same pre-existing comorbidities were also common among service members who died from COVID-19.
For each of the comorbidities evaluated in this study, hospitalized COVID-19 cases were more likely than non-hospitalized cases to have had a medical encounter for the condition in the past year. Chronic liver disease, hypertension, and chronic kidney disease were among the comorbidities most likely to be previously diagnosed in the hospitalized cases compared to the non-hospitalized cases. The finding that chronic liver disease was more likely to be diagnosed in hospitalized compared to non-hospitalized cases is noteworthy because recent studies have suggested that chronic liver disease is not a significant comorbid condition for COVID-19.16,17 However, other studies have indicated that cirrhosis increases risk for COVID-19 hospitalization and death among patients with chronic liver disease, and that cirrhosis may play a role in immune dysfunction.18,19
There were totals of 1,760 hospitalizations (0.9%) and 45 deaths among service members as of Aug. 31, 2021. Although there were 11,899 cases of COVID-19 occurring among fully vaccinated individuals, only 0.4% of hospitalized cases were fully vaccinated and no service member deaths occurred among fully vaccinated individuals, highlighting the importance of COVID-19 vaccination for force health protection. In particular, 45 deaths corresponds to a cumulative case fatality ratio of about 0.02% (i.e., 1 death per 4,205 infected), which is much lower than the case fatality ratio of 1.6% observed in the general U.S. population.20 This lower case fatality ratio is not surprising given that service members are on average younger and healthier compared to members of the general U.S. population.
It should be noted that the progression of illness followed a standard clinical course for a majority of service members who died from COVID-19 during the surveillance period.21 However, there were a few individuals who experienced rapid deterioration after having been discharged from the hospital and some who died suddenly at home or at an alternate site with COVID symptoms or a previously diagnosed COVID-19 infection. Acute respiratory and thrombotic complications of COVID-19 have been previously reported as common causes of death, with many patients having comorbidities such as hypertension, ischemic heart disease, and obesity.22 In patients who die suddenly, severe systemic inflammation, multi-organ dysfunction, and cardiovascular failure may also play a role.23–25 It is important to note that, although service members are a generally young and healthy population experiencing few deaths due to COVID-19, they are still vulnerable to the harmful effects of this disease particularly if they have pre-existing comorbidities and are unvaccinated.
There are several limitations that should be considered when interpreting the results of this study. First, the prevalence of pre-existing comorbidities among all cases may be underestimated because medical encounter data were queried in the year prior to the incident date of the case. Individuals who did not seek medical care for these conditions during this time period would not be captured. However, comorbidities may also be overestimated because the case definition allowed for only a single medical encounter to qualify as a comorbidity; therefore, screening encounters that were miscoded using the diagnosis code for that condition may overestimate the occurrence of certain pre-existing comorbidities. Importantly, as the science evolves more is being learned about the types of medical conditions that place individuals at higher risk for severe illness from COVID-19. The list of comorbidities investigated in this analysis is not exhaustive and should be re-evaluated in subsequent investigations. Second, the expansion of MHS Genesis to military treatment facilities throughout most of the western U.S. presented challenges to linking MHS GENESIS laboratory records with case information in the DRSi and the CHCS. As a result, some cases may have been missed. Finally, the number of cases, vaccination information, and hospitalization status may have also been underestimated if individuals were seen for care outside of the MHS TRICARE network, which is most likely to occur among inactivated reserve/guard members.
The COVID-19 pandemic continues to have persistent person-to-person spread in the community worldwide and will require continuous monitoring as new variants of the SARS-CoV-2 virus emerge. Findings presented here draw attention to the necessity of building on present-day disease surveillance efforts to collect and analyze case prevalence data, and in particular data including vaccination status, hospitalization, death, and serious underlying health conditions. Continued surveillance of COVID-19 among service members remains an important part of force health protection efforts.
References
- Johns Hopkins University Coronavirus Resource Center. Mortality Analyses. Updated 6 November 2021. Accessed 6 November 2021. https://coronavirus.jhu.edu/map.html
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology. The Epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;2(8):113–122.
- CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—U.S., February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346.
- Centers for Disease Control and Prevention. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—U.S., February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386.
- Centers for Disease Control and Prevention. People who are at increased risk for severe illness with certain medical conditions. Accessed 11 November 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- Polack FP, Thomas SJ, Kitchen N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615.
- Baden LR El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403–416.
- Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201.
- Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunizations Practises’ interim recommendation for use of the Janssen COVID-19 vaccine – United States, February 2021. MMWR. 2021;70(9):329–332.
-
1Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin Infect Dis. 2021;ciab079.
- Centers for Disease Control and Prevention. Delta Variant: What We Know About the Science. Accessed 11 November 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html.
- Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;s41591-021-01583-4.
- Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594.
- Tenforde MW, Self WH, Naioti EA, et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1156–1162.
- Department of Defense. Deputy Secretary of Defense. Memo: Mandatory coronavirus disease 2019 vaccination of Department of Defense service members. 21 August 2021. Accessed 11 November 2021. https://media.defense.gov/2020/Dec/08/2002548508/-1/-1/0/CORONAVIRUS-DISEASE-2019-VACCINE-GUIDANCE.PDF
- Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): A pooled analysis. Eur J Gastroenterol Hepatol. 2021;33(1):114–115.
- Lin, J., Bao, B., Khurram, N.A. et al. Chronic liver disease not a significant comorbid condition for COVID-19. Sci Rep. 2021;11(1):11734.
- Sharma P, Kumar A, Anikhindi S, et al. Effect of COVID-19 on Pre-existing Liver disease: What Hepatologist Should Know? J Clin Exp Hepatol. 2021;11(4):484–493.
- Marjot T, Webb GJ, Barritt AS 4th, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–364.
- Johns Hopkins University Coronavirus Resource Center. Mortality Analyses. Updated 11 November 2021. Accessed 11 November 2021. https://coronavirus.jhu.edu/data/mortality
- Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for In-Hospital Complications Associated with COVID-19 and Influenza - Veterans Health Administration, United States, October 1, 2018-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1528–1534.
- Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11(1):4263.
- Yang N, Tian K, Jin M, et al. Sudden death of COVID-19 patients in Wuhan, China: A retrospective cohort study. J Glob Health. 2021;11:05006.
- Polat V, Bostancı Gİ. Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J Thromb Thrombolysis. 2020;50(1):239–241.
- Giudicessi JR. Excess out-of-hospital sudden deaths during the COVID-19 pandemic: A direct or indirect effect of SARS-CoV-2 infections? Heart Rhythm. 2021;18(2):219–220.