Abstract
Malaria infection remains an important health threat to U.S. service members who are located in endemic areas because of long-term duty assignments, participation in shorter-term contingency operations, or personal travel. In 2018, a total of 58 service members were diagnosed with or reported to have malaria. This represents a 65.7% increase from the 35 cases identified in 2017. The relatively low numbers of cases during 2012–2018 mainly reflect decreases in cases acquired in Afghanistan, a reduction due largely to the progressive withdrawal of U.S. forces from that country. The percentage of cases of malaria caused by unspecified agents (63.8%; n=37) in 2018 was the highest during any given year of the surveillance period. The percentage of cases identified as having been caused by Plasmodium vivax (10.3%; n=6) in 2018 was the lowest observed during the 10-year surveillance period. The percentage of malaria cases attributed to P. falciparum (25.9 %) in 2018 was similar to that observed in 2017 (25.7%), although the number of cases increased. Malaria was diagnosed at or reported from 31 different medical facilities in the U.S., Afghanistan, Italy, Germany, Djibouti, and Korea. Providers of medical care to military members should be knowledgeable of and vigilant for clinical manifestations of malaria outside of endemic areas.
What Are the New Findings?
A total of 58 service members were diagnosed with or reported to have malaria in 2018 compared with 35 in 2017. Most of the malaria cases (63.8%) were caused by unspecified agents and were presumed to be acquired in Afghanistan (34.5%) or Africa (24.1%).
What Is the Impact on Readiness and Force Health Protection?
Service members are at risk of malaria infection via deployment or personal travel to endemic regions. Commanders should stress the importance of adherence to personal protective measures. Military health care providers in non-endemic regions should be aware of the signs and symptoms of malaria.
Background
Globally, the incidence rate of malaria is estimated to have decreased by 18% between 2010 and 2017, from 72 to 59 cases per 1,000 population at risk.1 However, for the second consecutive year, the World Health Organization reported a relative plateauing in the numbers of cases of malaria; in 2017, there were an estimated 219 million cases of malaria compared with 217 million in 2016.1 During the 6 years prior, the number of people contracting malaria globally had been steadily decreasing, from 239 million in 2010 to 214 million in 2015.1
A total of 87 countries reported indigenous malaria cases in 2017, with countries in Africa accounting for around 92% of worldwide malaria cases and 93% of all malaria-related deaths.1 The majority of these cases and deaths occurred in sub-Saharan Africa among children under 5 years of age and were due to mosquito-transmitted Plasmodium falciparum, but P. vivax, P. ovale, and P. malariae can also cause severe disease.1,2 Globally, 3.4% of estimated malaria cases are due to P. vivax; however, outside of the African continent, the proportion of P. vivax infections is 36.8%.1 In 2017, 82% of vivax malaria cases occurred in 5 countries including India, Pakistan, Ethiopia, Afghanistan, and Indonesia.1
Since 1999, the MSMR has published regular updates on the incidence of malaria among U.S. service members.3,4,5 The MSMR’s focus on malaria reflects both historical lessons learned about this mosquito-borne disease and the continuing threat that it poses to military operations and service members’ health. Malaria infected many thousands of service members during World War II (approximately 695,000 cases), the Korean War (approximately 390,000 cases), and the conflict in Vietnam (approximately 50,000 cases).6,7 More recent military engagements in Africa, Asia, Southwest Asia, the Caribbean, and the Middle East have necessitated heightened vigilance, preventive measures, and treatment of cases.8-16
In the planning for overseas military operations, the geography-based presence or absence of the malaria threat is usually known and can be anticipated. However, when preventive countermeasures are needed, their effective implementation is multifaceted and depends on the provision of protective equipment and supplies, individuals' understanding of the threat and attention to personal protective measures, treatment of malaria cases, and medical surveillance. The U.S. Armed Forces have long had policies and prescribed countermeasures effective against vector-borne diseases such as malaria, including chemoprophylactic drugs, permethrin-impregnated uniforms and bed nets, and topical insect repellents containing N,N-diethyl-meta-toluamide (DEET). When cases and outbreaks of malaria have occurred, they generally have been due to poor adherence to chemoprophylaxis and other personal preventive measures.9-12
MSMR malaria updates from the past 6 years documented that the annual case counts among service members after 2011 were the lowest in more than a decade.5,17-21 In particular, these updates showed that the numbers of cases associated with service in Afghanistan had decreased substantially in the past 6 years, presumably due to the dramatic reduction in the numbers of service members serving there. This update for 2018 uses methods similar to those employed in previous analyses to describe the epidemiologic patterns of malaria incidence among service members in the active and reserve components of the U.S. Armed Forces.
Methods
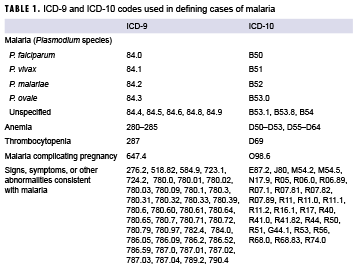
The surveillance period was 1 Jan. 2009 through 31 Dec. 2018. The surveillance population included Army, Navy, Air Force, and Marine Corps active and reserve component members of the U.S. Armed Forces. The records of the Defense Medical Surveillance System (DMSS) were searched to identify reportable medical events and hospitalizations (in military and nonmilitary facilities) that included diagnoses of malaria. A case of malaria was defined as an individual with 1) a reportable medical event record of confirmed malaria, 2) a hospitalization record with a primary diagnosis of malaria, 3) a hospitalization record with a non-primary diagnosis of malaria due to a specific Plasmodium species, 4) a hospitalization record with a non-primary diagnosis of malaria plus a diagnosis of anemia, thrombocytopenia and related conditions, or malaria complicating pregnancy in any diagnostic position, 5) a hospitalization record with a non-primary diagnosis of malaria plus diagnoses of signs or symptoms consistent with malaria (as listed in the Control of Communicable Diseases Manual, 18th Edition)22 in each diagnostic position antecedent to malaria, or 6) a positive malaria antigen test plus an outpatient record with a diagnosis of malaria in any diagnostic position within 30 days of the specimen collection date. The relevant ICD-9/ICD-10 codes are shown in Table 1. Laboratory data for malaria were provided by the Navy and Marine Corps Public Health Center.
This analysis allowed 1 episode of malaria per service member per 365-day period. When multiple records documented a single episode, the date of the earliest encounter was considered the date of clinical onset, and the most specific diagnosis recorded within 30 days of the incident diagnosis was used to classify the Plasmodium species.
Presumed locations of malaria acquisition were estimated using a hierarchical algorithm: 1) cases diagnosed in a malarious country were considered acquired in that country, 2) reportable medical events that listed exposures to malaria endemic locations were considered acquired in those locations, 3) reportable medical events that did not list exposures to malaria endemic locations but were reported from installations in malaria endemic locations were considered acquired in those locations, 4) cases diagnosed among service members during or within 30 days of deployment or assignment to a malarious country were considered acquired in that country, and 5) cases diagnosed among service members who had been deployed or assigned to a malarious country within 2 years prior to diagnosis were considered acquired in those respective countries. All remaining cases were considered acquired in unknown locations.
Results
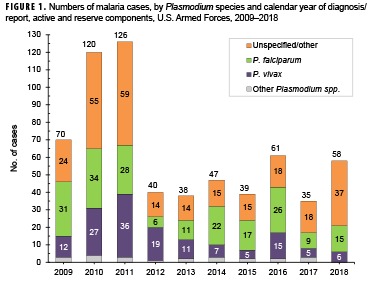
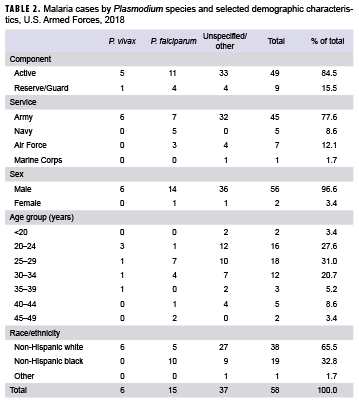
In 2018, a total of 58 service members were diagnosed with or reported to have malaria (Table 2). This represents a 65.7% increase from the 35 cases identified in 2017 (Figure 1). The percentage of cases of malaria caused by unspecified agents (63.8%; n=37) in 2018 was the highest during any given year of the surveillance period. Of all malaria cases identified, 6 (10.3%) were attributed to P. vivax and 15 (25.9%) to P. falciparum. In 2018, the percentage of malaria cases caused by P. vivax was the lowest percentage observed during the 10-year surveillance period. The percentage of malaria cases caused by P. falciparum in 2018 was comparable to that observed in 2017 (25.7%), although the number of cases increased. There were no cases identified as having been caused by P. malariae or P. ovale in 2018 (Figure 1).
Similar to 2017, the majority of U.S. military members diagnosed with malaria in 2018 were male (96.6%), active component members (84.5%), in the Army (77.6%), and in their 20s (58.6%) (Table 2).
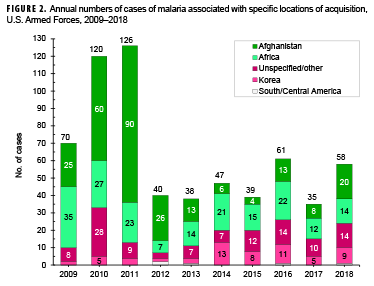
Of the 58 malaria cases in 2018, more than one-third (34.5%; n=20) of the infections were considered to have been acquired in Afghanistan, but almost one-quarter (24.1%; n=14) could not be associated with a known, specific location. Acquisition of the remaining cases was attributed mainly to Africa (24.1%; n=14) and Korea (15.5%; n=9), with 1 case (1.7%) in South/Central America (Figure 2). Of the 14 malaria infections considered acquired in Africa in 2018, 4 were linked to Niger, 3 to Cameroon, 2 each to Kenya, Ghana, and Djibouti, and a single case to an unknown country (data not shown).
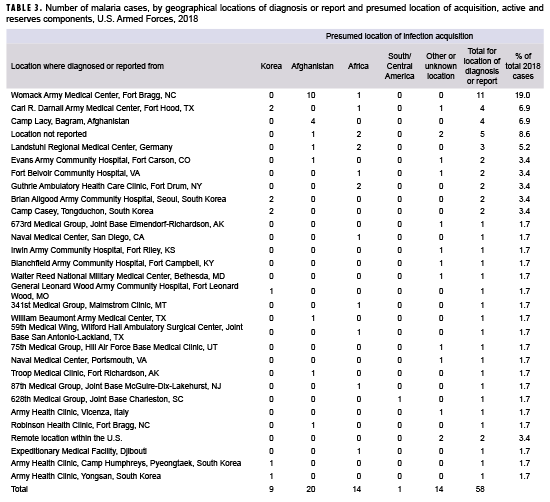
During 2018, malaria cases were diagnosed or reported from 31 different medical facilities in the U.S., Korea, Afghanistan, Germany, Italy, and Djibouti (Table 3). More than one-quarter (29.4%; 15/51) of the total cases with a known location of diagnosis were reported from or diagnosed outside the U.S., which represents a decrease from the 40.0% of malaria cases in this category in 2017. The largest number of malaria cases associated with a single medical facility during 2018 was 11 at the Womack Army Medical Center in Fort Bragg, NC.
In 2018, the percentage of malaria cases that were acquired in Africa (24.1%; n=14) was lower than the percentages of Africa-acquired cases observed in 2013 through 2017 (Figure 2). The percentage of Afghanistan-acquired cases (34.5%; n=20) in 2018 was the highest that it has been since 2012. The percentage of malaria cases acquired in Korea (15.5%; n=9) in 2018 was similar to percentages during 2016–2017 but slightly lower than those during 2014–2015 (Figure 2).
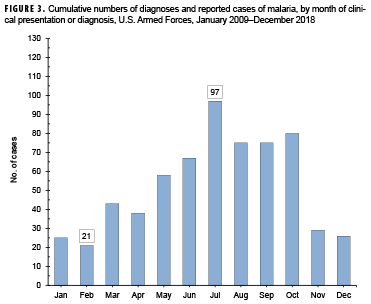
Between 2009 and 2018, the majority of malaria cases were diagnosed or reported during the 6 months from the middle of spring through the middle of autumn in the Northern Hemisphere (Figure 3). In 2018, 82.8% (48 of 58) of malaria cases among U.S. service members were diagnosed during May–Oct. (data not shown). This proportion is higher than the 71.3% (452/634) of cases diagnosed during the same 6-month intervals over the entire 10-year surveillance period. During 2009–2018, the proportions of malaria cases diagnosed or reported during May–Oct. varied by region of acquisition: Korea (91.9%; 57/62,); Afghanistan (80.0%; 212/265); Africa (60.0%; 114/190); and South/Central America (40.0%; 2/5) (data not shown).
Editorial Comment
MSMR annual reports on malaria incidence among all U.S. services began in 2007. The current report and those of the previous 5 years document that the lowest annual numbers of cases during the interval 2001–2017 were in the past 6 years,* reaching a nadir of 35 in 2017.5,17-21 The next lowest annual number of malaria cases occurred in 2013 (n=38). Most of the marked decline in the past 7 years is attributable to the decrease in numbers of malaria cases associated with service in Afghanistan. The dominant factor in that trend has undoubtedly been the progressive withdrawal of U.S. forces from that country.
This report also documents the fluctuating incidence of acquisition of malaria in Africa and Korea among U.S. military members during the past decade. Although the predominant species of malaria in Korea and Afghanistan has been P. vivax, the more dangerous P. falciparum species is of primary concern in Africa. The planning and execution of military operations on that continent must incorporate actions to counter the threat of infection by that potentially deadly parasite wherever it is endemic. The 2014–2015 employment of U.S. service members to aid in the response to the Ebola virus outbreak in West Africa is an example of an operation where the risk of P. falciparum malaria was significant.2 The finding that P. falciparum malaria was diagnosed in over one quarter of the cases in 2018 further underscores the need for continued emphasis on prevention of this disease, given its potential severity and risk of death.
The observations about the seasonality of diagnoses of malaria are compatible with the presumption that the risk of acquiring and developing symptoms of malaria in a temperate climatic zone of the Northern Hemisphere would be greatest during May–Oct. Given the typical incubation periods of malaria infection (approximately 9–14 days for P. falciparum, 12–18 days for P. vivax and P. ovale, and 18–40 days for P. malariae)22 and the seasonal disappearance of biting mosquitoes during the winter, most malaria acquired in Korea and Afghanistan would be expected to cause symptoms during the warmer months of the year. However, it should be noted that studies of P. vivax malaria in Korea have found that the time between primary infection and clinical illness among different P. vivax strains ranges between 8 days and 8–13 months and that as many as 40–50% of infected individuals may not manifest the symptoms of their primary illness until 6–11 months after infection.23 Klein and colleagues recently reported a cluster of 11 U.S. soldiers with P. vivax malaria who were likely infected at a training area located near the southern border of the demilitarized zone in 2015.24 Nine of the malaria cases developed their first symptoms of infection 9 or more months after exposure and after their departure from Korea.24 Transmission of malaria in tropical regions such as sub-Saharan Africa is less subject to the limitations of the seasons as in temperate climates but depends more on other factors affecting mosquito breeding such as the timing of the rainy season and altitude (below 2,000 meters).25
There are significant limitations to this report that should be considered when interpreting the findings. For example, the ascertainment of malaria cases is likely incomplete; some cases treated in deployed or non-U.S. military medical facilities may not have been reported or otherwise ascertained at the time of this analysis. Furthermore, it should be noted that medical data from military treatment facilities that are using MHS GENESIS are not available in DMSS, which was implemented at different sites throughout 2017. These include Naval Hospital Oak Harbor, Naval Hospital Bremerton, Air Force Medical Services Fairchild, and Madigan Army Medical Center. Therefore, the medical encounter data for individuals seeking care at 1 of these facilities were not captured in this analysis.
This MSMR report represents the first time that cases were included if they had a positive malaria antigen test plus an outpatient record with a diagnosis of malaria in any diagnostic position within 30 days of the specimen collection date. The relative accuracy of this revised case definition in estimating malaria incidence is corroborated by the results of a study in this issue of the MSMR.26 It is estimated that this modification of the case definition added about 10 cases over the 10-year surveillance period on top of the 624 cases that were identified in the reportable events and hospitalizations data. Diagnoses of malaria that were documented only in outpatient settings without records of a positive malaria antigen test and that were not reported as notifiable events were not included as cases. Also, the locations of infection acquisitions were estimated from reported relevant information. Some cases had reported exposures in multiple malarious areas, and others had no relevant exposure information. Personal travel to or military activities in malaria-endemic countries were not accounted for unless specified in notifiable event reports.
As in prior years, in 2018 most malaria cases among U.S. military members were treated at medical facilities remote from malaria endemic areas. Providers of acute medical care to service members (in both garrison and deployed settings) should be knowledgeable of and vigilant for the early clinical manifestations of malaria among service members who are or were recently in malaria-endemic areas. Care providers should also be capable of diagnosing malaria (or have access to a clinical laboratory that is proficient in malaria diagnosis) and initiating treatment (particularly when P. falciparum malaria is clinically suspected).
Continued emphasis on adherence to standard malaria prevention protocols is warranted for all military members at risk of malaria. Personal protective measures against malaria include the proper wear of permethrin-treated uniforms and the use of permethrin-treated bed nets, the topical use of military-issued DEET-containing insect repellent, and compliance with prescribed chemoprophylactic drugs before, during, and after times of exposure in malarious areas. Current Department of Defense guidance about medications for prophylaxis of malaria summarizes the roles of chloroquine, atovaquone-proguanil, doxycycline, mefloquine, and primaquine.27
*A recent MMWR Surveillance Summary reported the numbers of malaria cases among U.S. military personnel during 2009–2015, a period overlapping with the current analysis.5 However, because the MMWR analysis employed a malaria case definition different from that used in the current analysis, the numbers of annual cases differ.
Acknowledgements: The authors thank the Navy and Marine Corps Public Health Center, Portsmouth, VA, for providing laboratory data for this analysis.
References
- World Health Organization. World Malaria Report 2018. WHO, Geneva 2018. http://www.who.int/malaria/publications/world-malaria-report-2018/en/. Accessed on 15 Jan. 2018.
- Mace KE, Arguin PM, Tan KR. Malaria Surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(7):1–28.
- U.S. Army Center for Health Promotion and Preventive Medicine. Malaria, U.S. Army, 1998. MSMR. 1999;5(1):2–3.
- U.S. Army Center for Health Promotion and Preventive Medicine. Malaria experience among U.S. active duty soldiers, 1997–1999. MSMR. 1999;5(8):2–3.
- Armed Forces Health Surveillance Branch. Update: Malaria, U.S. Armed Forces, 2017. MSMR. 2018;25(2):2–7.
- Gupta RK, Gambel JM, Schiefer BA. Personal protection measures against arthropods. In: Chapter 22, Military Preventive Medicine: Mobilization and Deployment, Volume 1. Kelley, PW ed. Department of the Army, Office of the Surgeon General. Textbooks of Military Medicine. 2003:503–521.
- Ognibene AJ, Barrett, O. Malaria: Introduction and background. In: Internal Medicine in Vietnam (Vol II): General Medicine and Infectious Diseases. Ognibene AJ, Barrett O eds. Office of the Surgeon General, Center of Military History, U.S. Army; Washington, DC, 1982:271–278.
- Shanks GD, Karwacki JJ. Malaria as a military factor in Southeast Asia. Mil Med.1991;156(12):684–668.
- Kotwal RS, Wenzel RB, Sterling RA, et al. An outbreak of malaria in U.S. Army Rangers returning from Afghanistan. JAMA. 2005;293(2):212–216.
-
1Whitman TJ, Coyne PE, Magill AJ, et al. An outbreak of Plasmodium falciparum malaria in U.S. Marines deployed to Liberia. Am J Trop Med Hyg. 2010;83(2):258–265.
- Centers for Disease Control and Prevention. Malaria acquired in Haiti–2010. MMWR. 2010;59(8):217–218.
- Shaha DP, Pacha LA, Garges EC, Scoville SL, Mancuso JD. Confirmed malaria cases among active component U.S. Army personnel, Jan.–Sept. 2012. MSMR. 2013;20(1):6–9.
- Lee JS, Lee WJ, Cho SH, Ree H. Outbreak of vivax malaria in areas adjacent to the demilitarized zone, South Korea, 1998. Am J Trop Med Hyg. 2002;66(1):13–17.
- Armed Forces Health Surveillance Center. Korea-acquired malaria, U.S. Armed Forces, Jan. 1998–Oct. 2007. MSMR. 2007;14(8):2–5.
- Ciminera P, Brundage J. Malaria in U.S. military forces: A description of deployment exposures from 2003 through 2005. Am J Trop Med Hyg. 2007;76(2):275–279.
- Armed Forces Health Surveillance Center. Malaria among deployers to Haiti, U.S. Armed Forces, 13 Jan.–30 June 2010. MSMR. 2010;17(8):11.
- Armed Forces Health Surveillance Center. Update: Malaria, U.S. Armed Forces, 2012. MSMR. 2013;20(1):2–5.
- Armed Forces Health Surveillance Center. Update: Malaria, U.S. Armed Forces, 2013. MSMR. 2014;21(1):4–7.
- Armed Forces Health Surveillance Center. Update: Malaria, U.S. Armed Forces, 2014. MSMR. 2015;22(1):2–6.
- Armed Forces Health Surveillance Branch. Update: Malaria, U.S. Armed Forces, 2015. MSMR. 2016;23(1):2–6.
- Armed Forces Health Surveillance Branch. Update: Malaria, U.S. Armed Forces, 2016. MSMR. 2017;24(1):2–6.
- Heymann DL, ed. Control of Communicable Diseases Manual, 18th Edition. Washington, DC: American Public Health Association; 2004.
- White, NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011 (10):297.
- Klein TA, Seyoum B, Forshey BM, et al. Cluster of vivax malaria in U.S. soldiers training near the demilitarized zone, Republic of Korea during 2015. MSMR. 2018;25(11):4–9.
- Fairhurst RM, Wellems TE. Plasmodium species (malaria). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Mandell GL, Bennett JE, Dolin R eds. 7th Edition. Churchill Livingstone Elsevier. 2010.
- O'Donnell FL, Mancuso JD, Stahlman S. Re-evaluation of the MSMR case definition for incident cases of malaria. MSMR. 2019;26(2):8–14.
- Assistant Secretary of Defense for Health Affairs. Subject: Guidance on Medications for Prophylaxis of Malaria. HA-Policy 13-002. 15 April 2013.