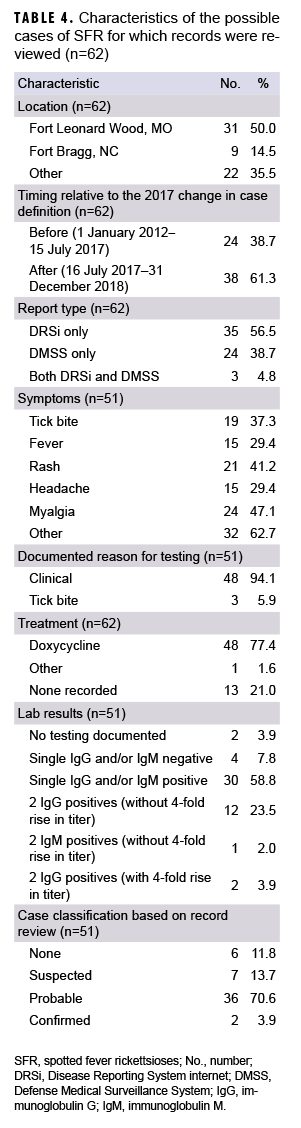
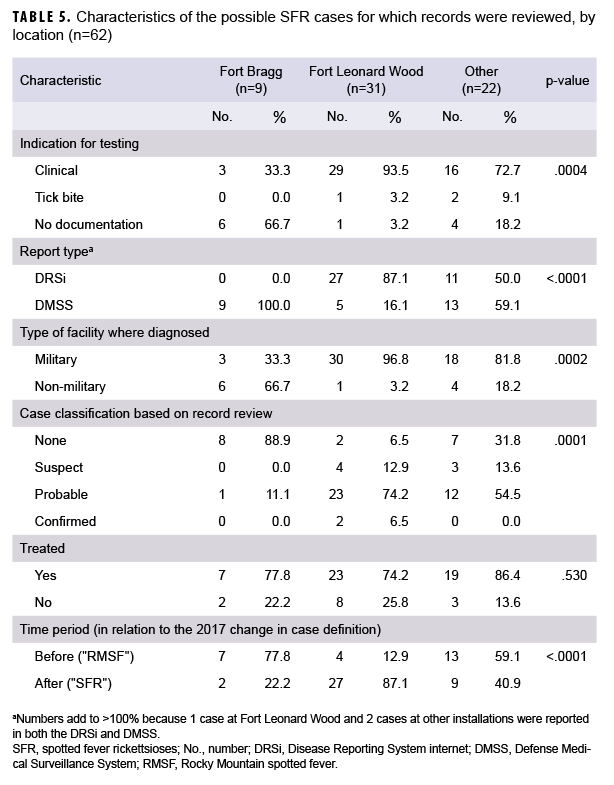
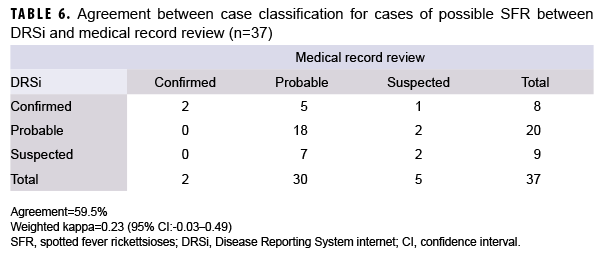
What Are the New Findings?
Cases of SFR have been increasing in U.S. civilian and military populations since the mid-1990s. In 2017 and 2018, nearly 80% of military beneficiaries diagnosed with SFR received presumptive treatment despite a lack of symptoms consistent with SFR and only 4% had confirmed disease.
What Is the Impact on Readiness and Force Health Protection?
Service members may be at increased risk of contracting SFR because of residence in regions with endemic rickettsial species and frequent field training in tick habitats. However, military health providers should adhere to national guidelines for diagnosis, treatment, and use of modern laboratory methods to confirm the diagnosis.
Abstract
Spotted fever rickettsioses (SFR) are emerging in the Atlantic and Central regions of the U.S., though cases have been reported across the contiguous U.S. Military populations may be at increased risk for SFR because of residence in these regions and frequent field training in tick habitats. Surveillance for Rocky Mountain spotted fever in the Army began in 1998 and was expanded to include all SFR in 2017. Between 2016 and 2017, the rate of active component cases reported from Army installations in the Atlantic and Central regions of the U.S. increased nearly five-fold from 2016 (0.55 per 100,000 person-years [p-yrs]) to 2017 (2.65 per 100,000 p-yrs). The majority of SFR cases were reported from Fort Leonard Wood, MO, and Fort Bragg, NC. Most reported cases had no documented symptoms consistent with SFR and could not be confirmed as "cases" by standard case-defining methods. SFR surveillance and control efforts in military populations can be improved by better adherence to guidelines for SFR diagnosis and through the use of available advanced laboratory techniques.
Background
Spotted fever rickettsioses (SFR) can cause human infections ranging from asymptomatic or mild cases to severe, life-threatening disease.1–3 SFR infections are caused by bacterial species of the genus Rickettsia. For example, SFR in the U.S. are caused by R. rickettsii (Rocky Mountain spotted fever [RMSF]), R. parkeri (R. parkeri rickettsiosis), and R. species 364D (Pacific Coast tick fever).2 These bacterial species are transmitted to humans through the bite of an infected tick, most commonly the American dog tick, Rocky Mountain wood tick, and brown dog tick.2 These vectors have varying geographic distributions across the U.S. and can be found in regions where Army installations are present. Additionally, those traveling outside the U.S. may be at risk for other tick-borne rickettsial diseases, including African tick-bite fever, Mediterranean spotted fever (boutonneuse fever), Queensland tick typhus, Japanese spotted fever, and others.4,5 Early treatment with doxycycline typically results in rapid recovery, but after the fifth day of illness, untreated cases have a higher risk of developing severe illness, including disseminated intravascular coagulopathy and mulisystem organ failure, potentially leading to permanent disabilities or death.1–3
The incidence of tick-borne diseases, including SFR, has been increasing in the U.S.1 The highest number of cases of tick-borne disease in the past 14 years was reported to the Centers for Disease Control and Prevention (CDC) in 2017.6 Annual incidence of SFR in the U.S. increased from 1.7 cases per 1 million persons in 2000 to 13.2 cases per 1 million persons in 2016.6 Cases have been reported from throughout the contiguous U.S.; SFR are not reportable conditions in Hawaii or Alaska.6 From 2008–2012, 63% of cases were reported to CDC from 5 states: Arkansas, Missouri, North Carolina, Oklahoma, and Tennessee.7,8 In Missouri, the state in which Fort Leonard Wood is located, the incidence rate of RMSF during 1 Jan. 2019–2 July 2019 was 32.7 cases per 1 million persons.9 An overall seasonality has been recognized throughout most of the U.S., with 68% of cases reporting symptom onset occurring between May and Sept. and the highest number of cases in June.
CDC included RMSF as a notifiable condition in the National Notifiable Diseases Surveillance System from 1944 to 2009.10 However, the SFR are antigenically related and serologic assays developed for the diagnosis of RMSF may react nonspecifically with antigens of less pathogenic species.11 For this reason, the Council of State and Territorial Epidemiologists changed the case definition of RMSF to the broader category called SFR. This reporting change, implemented by the states and CDC in 2010, may account for some of the increase in SFR reported to CDC in recent years, although the upward trend in disease incidence began much earlier in the 1990s.7 Because of limitations of laboratory diagnostic evidence used to identify cases, the epidemiology of all spotted fever group rickettsioses remains unclear.1,2,11
Military populations may be at increased risk for SFR because of residence in endemic regions and frequent field training in tick habitats.12–14 Further, those in a military occupational specialty that included ground combat have been found to be more likely to be seropositive for rickettsiosis.12–14 Surveillance for rickettsial disease in the U.S. military began in 1998, but it only included cases of RMSF. 15,16 In July 2017, the Armed Forces broadened the reportable medical event guidelines and case definitions to include all SFR, consistent with CDC surveillance.16 However, the clinical criteria of these 2 case definitions differ in that the Armed Forces definition did not require the presence of fever to meet the confirmed or probable case definition.
Given the potential for severe disease presentation, the changing epidemiology in the U.S., and the potential for service-related risk, surveillance for SFR is critical to informing Department of Defense (DOD) efforts in force health protection risk assessment, mitigation, and communication. However, the recent epidemiology of SFR among military populations has not been evaluated. Furthermore, public health personnel at Fort Leonard Wood had been reporting concerns about high rates of SFR at that installation since the 2017 change in surveillance. The primary goal of this study was to characterize the epidemiology of the SFR among active component (AC) Army service members and other beneficiaries assigned to Army installations in the Central and Atlantic regions of the U.S. from 2012 to 2018, with a special focus on Fort Leonard Wood, MO. The secondary goal of the study was to assess the completeness and accuracy of SFR reporting at these installations.
Methods
Study population
The study population included all AC and other U.S. military beneficiaries assigned to all of the Army installations in the Atlantic and Central regions of the U.S. between 1 Jan. 2012 and 31 Dec. 2018. Installations in the Pacific and European regions were excluded from the study because of differences in climate, geography, and disease epidemiology.
Case definition
Cases were classified according to the 2017 Armed Forces Reportable Medical Event Guidelines and Case Definitions.16 The clinical criteria for SFR included a patient with any of the following: rash, headache, myalgia, nausea/vomiting, anemia, thrombocytopenia, an ulcer at the site of the bite, or any hepatic transaminase elevation. A confirmed case was defined as a case that met the clinical criteria along with any of the following: 1) 4-fold increase in immunoglobulin G (IgG) antibody titer, 2) DNA detected by polymerase chain reaction (PCR) from a clinical specimen, 3) histopathologic identification from a biopsy, or 4) identification by culture from a clinical specimen. A probable case was defined as a case that met the clinical criteria along with a single serum specimen positive for SFR immunoglobulin M (IgM) or IgG. A suspected case was defined as a case that met any of the laboratory criteria above but none of the clinical criteria.
Data sources
The primary data sources used in this study were the Disease Reporting System internet (DRSi), the Defense Medical Surveillance System (DMSS), the Armed Forces Health Longitudinal Technology Application (AHLTA), and laboratory data.17,18 The DMSS database was queried for records of care at or near Army installations in the Atlantic and Central regions (as indicated by the patient zip code) if the records contained International Classification of Diseases, 9th Revision code of 082.0 or International Classification of Diseases, 10th Revision code of A77.0 in any diagnostic position. All SFR cases reported to the DRSi are validated by epidemiologists the next day. Laboratory tests with an IgG or IgM value of ≥1:64 by indirect immunofluorescence antibody (IFA) for SFR were considered positive; an IgG or IgM value of 1:64 is the lowest value that can be interpreted as a positive result.
Additional data on cases reported from Fort Leonard Wood, including clinical and laboratory details of each case, were collected from 2017–2018 by the Fort Leonard Wood Preventive Medicine Department. This department reviews records from the laboratory, hospital, and clinics for every case of tick-borne disease and performs additional investigation for each case to determine its case classification and exposure history. Information was collected on the laboratory type, result, symptoms, tick exposure history, and treatment.
Extensive record reviews were also performed at the Armed Forces Health Surveillance Branch (AFHSB) using ALHTA for 62 randomly selected cases that had either been reported to the DRSi or clinically diagnosed and found in the DMSS between 2012 and 2018 at these installations. The reviews covered clinicians' documentation of medical encounters, medication records, and laboratory records. Case classifications based on the Armed Forces case definition were compared to the classification entered by the original DRSi reporter to identify inconsistencies.16
Statistical analysis
An individual was counted once per calendar year during the study period, and the earliest of the test order date (laboratory data), diagnosis date (DMSS), or report date (DRSi) in each year was used to determine when the case occurred. Cases could appear in more than 1 data source. If cases were found in multiple data sources, their records were matched by social security number and approximate date of test, DRSi report, or diagnosis. Duplicate or follow-up cases were removed based on social security number and date. An incidence rule of 365 days was implemented for laboratory data after matching cases by social security number to remove follow-up laboratory tests. Incidence rates were calculated using person-years (p-yrs) in the AC population from the DMSS. Because information on time at risk was not available for non-AC beneficiaries (e.g., the start and end dates of active duty service periods of reserve component members), rates were not calculated for these populations. Agreement between case classification recorded in the DRSi and that obtained from record review was measured by percent agreement and weighted kappa, which allows for partial agreement between the ordinal classification groups. All analyses were conducted using SAS/STAT software, version 9.4 (2014, SAS Institute, Cary, NC).
Results
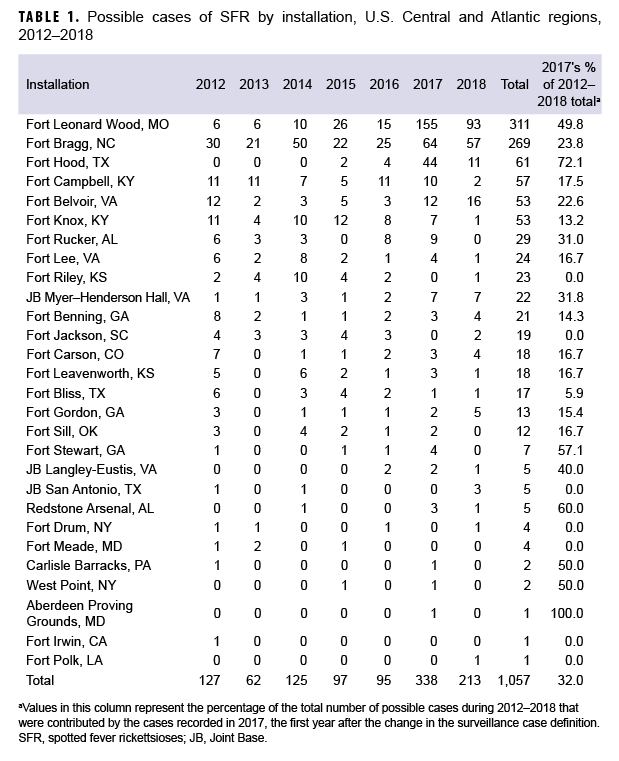
Installations located in 3 (Missouri, North Carolina, and Oklahoma) of the 5 states that reported the greatest numbers of possible cases to CDC during 2008–2012 included Fort Leonard Wood, MO; Fort Bragg, NC; and Fort Sill, OK. Two installations in Kentucky (Fort Campbell, KY and Fort Knox, KY) were used as proxies for Tennessee. Taken together, these 5 installations accounted for 702 (66.4%) of the total possible cases identified in the study (Table 1). No cases were reported from the DOD in Arkansas. At Fort Leonard Wood, the rates of possible cases in the AC population increased approximately 40-fold from before (12.4 per 100,000 p-yrs) to after (502.5 per 100,000 p-yrs) the change in case definition (data not shown). The increase in rates associated with the definition change was 3,952.4%. At Fort Bragg, rates approximately doubled from before (39.1 per 100,000 p-yrs) to after (78.9 per 100,000 p-yrs) the case definition change (data not shown).
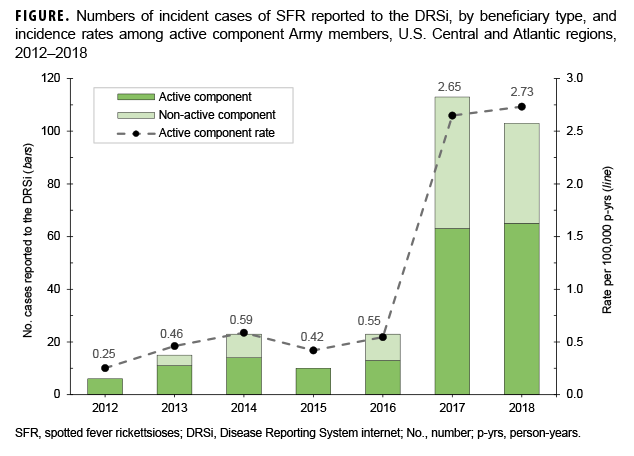
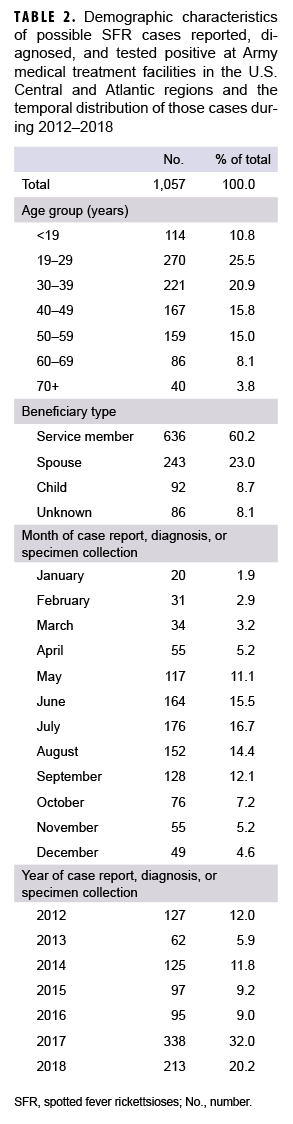
From 1 Jan. 2012–31 Dec. 2018, a total of 1,057 possible cases were captured from either DRSi case reports, DMSS diagnosed cases, the laboratory database, or a combination of the 3 (Table 2). Of the 1,057 possible cases, there were 1,046 unique individuals with just 1 SFR event; 8 people had repeat infections, 2 had DRSi case reports but did not have an identifying social security number, and 1 had more than 1 repeat infection of SFR per year (data not shown). Similar to civilian trends, possible cases most frequently occurred during June–Sept., with the highest number of possible cases occurring in July (n=176; 16.7%). Of the 1,057 possible cases, 25.5% were between 19–29 years of age and 20.9% were between 30–39 years of age. The greatest number of possible cases occurred in 2017 (n=338), a 255.8% increase from 2016 (n=95). Throughout the study period, the majority of possible cases were among AC service members (n=636; 60.2%) compared to other beneficiary types (n=421; 39.8%). The rate of cases reported to the DRSi among the AC population increased by 381.8% from 2016 through 2017, then increased by 3.0% between 2017 and 2018, for a net increase of 396.4% between 2016 and 2018 (Figure).
Fort Leonard Wood record review
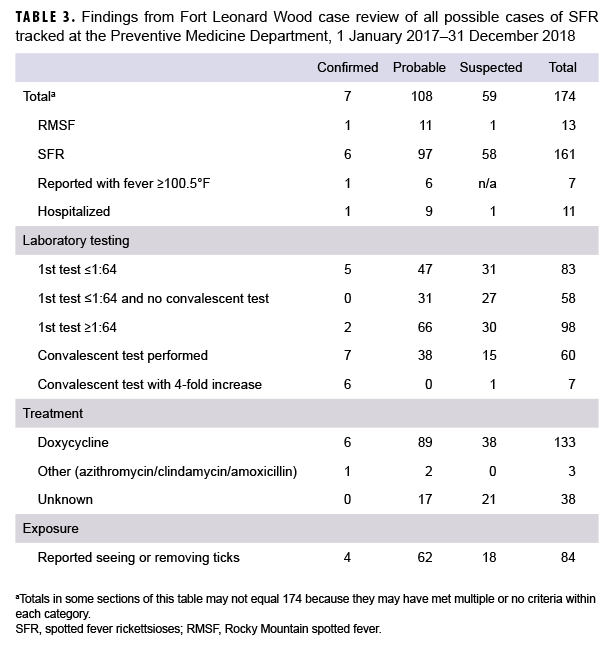
The Preventive Medicine Department at Fort Leonard Wood tracked all cases that tested positive for SFR in 2017 and 2018 (Table 3). Of the 174 cases tracked in these years, 7 were classified as confirmed (1 RMSF and 6 SFR), 108 were probable (11 RMSF and 97 SFR), and 59 were suspected (1 RMSF and 58 SFR). Of the 7 confirmed cases reported to the DRSi after the change in case definition, only 1 experienced a fever ≥100.5°F. A total of 11 cases (6.3%) were hospitalized; those hospitalized were mostly reported as probable (n=9; 81.8%). All antibody titers were performed using IFA assays; other serum antibody tests do not provide quantitative titers. A total of 83 (47.7%) cases had an antibody titer below the threshold of 1:64 for their first test (indicating a negative antibody result), and 58 (33.3%) reported cases had a negative acute antibody test and no convalescent test performed. These cases instead had a positive R. typhi or unidentifiable rickettsia antibodyIgM or IgG that the health care provider determined was clinically compatible. A total of 60 (34.5%) cases had a convalescent sera test performed, of which 7 (11.7%) showed at least a 4-fold increase in antibody titer. The average number of days between acute and convalescent sera was 28 days (median 19 days) (data not shown). Of the 174 reported cases, 136 (78.2%) received antibiotic treatment for SFR. It could not be determined if any antibiotic treatment was prescribed for 38 cases (21.8%). Nearly half (n=84; 48.3%) of cases tracked from 2017–2018 reported recently seeing or removing ticks from their bodies.
AFHSB record review
Record reviews were completed at AFHSB using AHLTA for 62 randomly selected cases from either DRSi or DMSS data with diagnosis dates from 2014–2018. Only 2 of the 51 cases (3.9%) diagnosed at military treatment facilities (MTFs) met the case definition for a confirmed case of SFR, both of which were diagnosed at Fort Leonard Wood (Table 4). Of the 36 possible cases that met the case definition for probable SFR (clinical symptoms plus an initial positive test for IgM or IgG antibody), only 15 had a follow-up test (convalescent) for antibody. Of those 15 with acute and convalescent tests, 13 had subsequent titer results that indicated an old infection rather than a new infection caused by recent exposure to the bacteria (data not shown). Thus, only 2 of the 15 (13.3%) who had paired testing appeared to be recent cases. Most cases (n=49; 79.0%) were treated presumptively despite lack of evidence of recent infection; empirical treatment of cases that did not show evidence of recent infection did not vary significantly by location. Among the cases treated presumptively, a tick bite alone as the reason for treatment was documented in only 6.1% (n=3) of chart reviewed cases. In cases with any documented symptoms, a tick bite was discussed in 37.3% of cases (n=19). In general, the recognized reason for testing in the 62 reviewed cases was based on clinical indication of symptoms (n=51; 82.3%), but the clinical symptoms were often inconsistent with those expected from SFR (Table 5). For example, indications included chronic low back pain, chest pain, multiple sclerosis, lupus, paresthesia, and fatigue. Two-thirds of diagnosed cases from Fort Bragg were from non-MTFs for which medical records were not available for review. In contrast, at Fort Leonard Wood almost all cases (n=30; 96.8%) were diagnosed at the MTF. At the other locations considered in this analysis, most SFR cases were diagnosed at MTFs (n=18; 81.8%) (Table 5).
In general, the recognized reason for testing in the 62 reviewed cases was based on clinical indication of symptoms (n=51; 82.3%), but the clinical symptoms were often inconsistent with those expected from SFR (Table 5). For example, indications included chronic low back pain, chest pain, multiple sclerosis, lupus, paresthesia, and fatigue. Two-thirds of diagnosed cases from Fort Bragg were from non-MTFs for which medical records were not available for review. In contrast, at Fort Leonard Wood almost all cases (n=30; 96.8%) were diagnosed at the MTF. At the other locations considered in this analysis, most SFR cases were diagnosed at MTFs (n=18; 81.8%) (Table 5).
After reviewing the cases' medical records, an assessment of the appropriate case classification status based on the Armed Forces reportable medical event (RME) case definition was determined. Of the 62 records reviewed, 37 had case reports in the DRSi to allow assessment of reliability; the other 25 had either no DRSi record (n=24) or did not report the case classification in the DRSi (n=1) (Table 6). Agreement between the information recorded and classified in the DRSi and the record review of cases was poor, at 59.5% agreement (22 of 37) (weighted kappa=0.23; 95% confidence interval: -0.03–0.49) (Table 6).
Editorial Comment
After expanding surveillance requirements in 2017 from only RMSF to the broader SFR group, there was a substantial increase in the numbers of diagnoses and reports of rickettsial diseases at U.S. Army installations in the Central and Atlantic regions (Figure). The largest proportions of these cases were seen at Fort Leonard Wood and Fort Bragg. A smaller increase in incidence was also seen in the civilian population but was part of an increasing national trend that has continued since the mid-1990s.7 In contrast, this report shows Army incidence increased only in the period from 2017 through 2018, suggesting a substantial surveillance bias effect. While only 4% of cases met the case definition for a confirmed case in the medical record review, this is consistent with the 1–7% of cases that were confirmed nationally in CDC estimates.2,6 Many patients lacked documented symptoms consistent with the diagnosis of SFR, and only 13.3% of the patients who had paired testing had evidence of acute disease. Additionally, agreement between the DRSi and record reviews was poor (agreement=59.5%; weighted kappa=0.23). These factors suggest a considerable amount of overdiagnosis and related overreporting of cases, and potentially unnecessary treatment.
Recent passive surveillance for SFR cases in the U.S. suggests that the severity of illness in humans has decreased for unknown reasons.1 Dahlgren and colleagues hypothesized that less pathogenic rickettsiae are causing human infections, while the incidence of disease caused by more pathogenic rickettsiae, such as R. rickettsii, has remained relatively stable over the years.1 Because of limitations in laboratory testing techniques across the U.S. and cross-reactivity of rickettsial species, this hypothesis has not yet been proven.1–3,11,14,19 In U.S. surveillance, the number of cases reported annually increased from 1,617 in 2010 to 2,275 in 2015, yet the percentage of confirmed cases with supportive laboratory evidence decreased from 1.9% in 2010 to 0.7% in 2015.2 Delisle and colleagues suggest that species other than R. rickettsii may be the most common rickettsial infections in Tennessee.19 Without more advanced laboratory testing in human cases to evaluate the spread of emerging pathogens in ticks, these hypotheses cannot be validated.
In this review, all laboratory tests performed at MTFs for SFR were IFA and other antibody tests; no records of testing with PCR of blood or eschar specimens were found. The diagnosis of SFR can be confirmed by paired IFA IgG serum specimens separated by 2–4 weeks, but this often does not result in a species-specific diagnosis. Diagnosis through molecular methods (i.e., real-time PCR) can both confirm the diagnosis in 1 sample and also offer species-specific diagnosis, which assists in understanding and controlling rickettsial diseases in military populations and supports force health protection efforts.2 The Naval Infectious Disease Diagnostic Laboratory (NIDDL) is certified to perform molecular diagnostic testing for rickettsial diseases on blood and eschars. Additionally, since 2018, real-time molecular assays have been made available to qualified state and local laboratories through CDC's Laboratory Response Network.2 In cases of suspected rickettsiosis, military health care providers should consider sending out laboratory specimens to both the NIDDL and their state health department for molecular testing. Use of these improved testing methods would not only improve adherence to CDC recommendations for clinical testing, but it would also enhance force health protection and readiness by improving public health surveillance. Such specific testing results would allow better targeting of prevention and control efforts and a better understanding of the expanding geographic distribution of the diseases carried by ticks.
The main limitation of this study is the difficulty in distinguishing true increases in SFR disease incidence from increases due to misclassification (surveillance) bias. The sudden increase in 2017 coincident with the change in case definition, however, strongly suggests a surveillance bias. IgG antibodies can remain elevated for months or years following exposure and subsequent clinical recovery from illness.2,11 National studies have estimated antibody seroprevalence in the U.S. population to be between 6%–22%, the latter for persons living in endemic regions.2 Many of those with positive acute titers may represent antibody persistence rather than recent infection.3 Since it is not possible to differentiate acute illness from previous infection using a single elevated IgG titer, the inclusion of cases based on single elevated IgG titers overestimates disease incidence. Further, IFA assays are insensitive during the early stages of infection when most patients seek medical attention; therefore, the low proportion of possible cases who are tested with paired samples may lead to underestimates of the risk of infection. Other limitations of this study include the relatively small sample size of charts that were reviewed and the inability to generalize findings to the civilian population or other locations where U.S. military forces are stationed.
The discrepancies seen between diagnostic trends among civilian and military providers at specific installations also suggest the need for better communication of risk between these providers at the installation level. For example, the lack of documentation of SFR diagnoses in AHLTA and the DRSi at Fort Bragg suggests different diagnostic practices between MTFs and non-MTFs, which may indicate a significant gap in disease surveillance. As more installations reduce their medical treatment capabilities, the gaps between military and civilian providers may become more significant across the DoD. Preventive medicine departments at military installations should position themselves to establish regular communications with civilian health departments and health care facilities frequented by military beneficiaries in order to have better awareness of disease burden in their population.
This study suggests the need for additional education of health care providers about CDC guidelines for diagnosis and treatment to reduce unnecessary treatment and increase confirmatory testing. CDC guidelines state that treatment is not indicated for an asymptomatic tick bite or an isolated IgG positive lab test, both of which were seen in this study. While CDC guidelines support the use of empiric therapy with doxycycline because of the nonspecific symptoms of rickettsial disease, providers appeared to be giving treatment to many patients whose symptoms are inconsistent with the diagnosis. For example, SFR testing was commonly included in a broad battery of testing in patients with longstanding, chronic illnesses, such as lupus, multiple sclerosis, or low back pain. In these cases, empiric therapy was not provided at time of testing, likely because of the low probability of infection. With that in mind, providers should avoid unnecessary treatment of chronic illnesses inconsistent with SFR infection while also continuing to follow CDC guidelines to initiate immediate treatment in patients with acute signs and symptoms that are consistent with SFR infection without waiting for laboratory confirmation.5 Despite the findings of potential case misclassification and overdiagnosis and treatment, this report endorses the continued need for robust prevention, detection, and response capabilities at all installations in risk areas. These measures include ensuring leaders are aware of the need to adhere to the DoD repellent system, to practice tick avoidance, and to promote awareness of the high-risk season and high-risk areas for tick exposure. Medical and public health assets should communicate the risk of SFR, its mitigation, and prevention to commanders, service members, and other beneficiaries. Practices that reduce tick bites can also reduce the risk of other arthropod-borne diseases as well as nuisance bites and other conditions that detract from daily readiness.
Future studies could evaluate the knowledge and implementation of tick-borne disease prevention practices in service members as well as the knowledge, skills, and behaviors of military providers regarding vector-borne disease indications, best testing practices, And proper treatment protocols. Simultaneously, public health assets must ensure that surveillance data are valid, complete, and communicated in a timely manner in order to allow for public health action and force health protection in their population. All SFR cases must be reported to both military and state/local health departments to ensure that requirements are met and that proper control measures are in place. Finally, agreement between the DRSi and record review classification of cases was poor and suggests an area for improvement by more thorough review and analysis of case reports.
Author affiliations: U.S. Army Public Health Center, Aberdeen Proving Ground, MD (Ms. Kebisek, Ms. Scatliffe-Carrion, Dr. Ambrose); Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD (COL Mancuso); Epidemiology and Disease Surveillance, U.S. Army Public Health Command Central Region, Joint Base San Antonio-Fort Sam Houston, TX (Dr. Stidham); Preventive Medicine Division, Fort Leonard Wood, MO (Ms. Doyel and MAJ Rice)
Disclaimer: The contents, views, or opinions expressed in this publication are those of the author(s) and do not necessarily reflect the official policy or position of the Defense Health Agency or the Department of Defense.
Acknowledgements: The authors thank Sara Bazaco, PhD, MPH, Armed Forces Health Surveillance Branch for assistance with obtaining laboratory and DMSS data, and COL Laura Pacha, MD, MPH, Regional Health Command-Central and COL Sheryl A Bedno, MC, Director of Public Health at Fort Bragg their review of this study.
References
- Dahlgren FS, Behravesh CB, Paddock CD, Eisen RJ, Springer YP. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg. 2016;94(1):35–42.
- Binder AM, Heitman KN, Drexler NA. Diagnostic methods used to classify confirmed and probable cases of spotted fever rickettsioses—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2019;68(10):243–246.
- Yoshimizu MH, Billeter S. Suspected and confirmed vector-borne rickettsioses of North America associated with human diseases. Trop Med Infect Dis. 2018;3(1):2.
- Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34(suppl 4):145–169.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65(2):1–44.
- Centers for Disease Control and Prevention. Lyme and other tickborne diseases increasing. https://www.cdc.gov/media/dpk/diseases-and-conditions/lyme-disease/index.html. Accessed 29 June 2020.
- Centers for Disease Control and Prevention. Epidemiology and statistics. https://www.cdc.gov/rmsf/stats/index.html. Accessed 29 June 2020.
- Drexler NA, Dahlgren FS, Heitman KN, Massung RF, Paddock CD, Behravesh CB. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):26–34.
- Missouri Department of Health & Senior Services. Communicable disease surveillance. https://health.mo.gov/living/healthcondiseases/communicable/communicabledisease/. Accessed 29 June 2020.
- Centers for Disease Control and Prevention. Spotted fever rickettsiosis (Rickettsia spp.) 2010 case definition. https://wwwn.cdc.gov/nndss/conditions/spotted-fever-rickettsiosis/case-definition/2010/. Accessed 29 June 2020.
-
Mcquiston JH, Wiedeman C, Singleton J, et al. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am J Trop Med Hyg. 2014;91(4):767–770
-
Goddard J, McHugh C. Impact of a severe tick infestation at Little Rock AFB, Arkansas on Volant Scorpion military training. Mil Med. 1990;155(6):277–280.
-
Graf PCF, Chretien J-P, Ung L, Gaydos JC, Richards AL. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin Infect Dis. 2008;46(1):70–77.
-
Jiang J, Myers TE, Rozmajzl PJ, et al. Seroconversions to rickettsiae in US military personnel in South Korea. Emerg Infect Dis. 2015;21(6):1073–1074.
-
Headquarters, Department of the Army. Army Regulation 40-5. Medical Services. Preventive Medicine. 25 May 2007.
-
Armed Forces Reportable Medical Events. https://wwwn.cdc.gov/nndss/conditions/spotted-fever-rickettsiosis/case-definition/2010/. 1 Jan. 2020. Accessed 16 June 2020.
-
Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense Serum Repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92(12):1900–1904.
-
Navy and Marine Corps Public Health Center, EpiData Center Department. Description of the MHS Health Level 7 Microbiology Laboratory for Public Health Surveillance. Jan. 2012.
-
Delisle J, Mendell NL, Stull-Lane A, Bloch KC, Bouyer DH, Moncayo AC. Human infections by multiple spotted fever group rickettsiae in Tennessee. Am J Trop Med Hyg. 2016;94(6):1212–1217.
-
Ambrose JF, Kebisek J, Gibson KJ, White DW, O’Donnell FL. Commentary: Gaps in reportable medical event surveillance across the Department of the Army and recommended training tools to improve surveillance practices. MSMR. 2019;26(8):17–21.