Common respiratory viruses are associated with substantial morbidity within the Military Health System. In 2022 more than 250,000 medical encounters were recorded within the MHS for respiratory illness among active component service members, with an additional 160,000 encounters for COVID-19.1 Among non-service member beneficiaries, there were approximately 2 million encounters for respiratory infections in 2022, with just over one quarter of these among the pediatric 0-17 years age group.2 During the most recent winter season in the Northern Hemisphere, the co-circulation at relatively high levels of influenza, SARS-CoV-2, and RSV was termed the “tripledemic.”3
Persons at high risk, especially those over 65 years of age, can be hospitalized or die from complications of infection with influenza, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), respiratory syncytial virus, or from other frequently encountered pathogens. Mortality from respiratory viral illness also occurs among infants, children, and adolescents.4,5
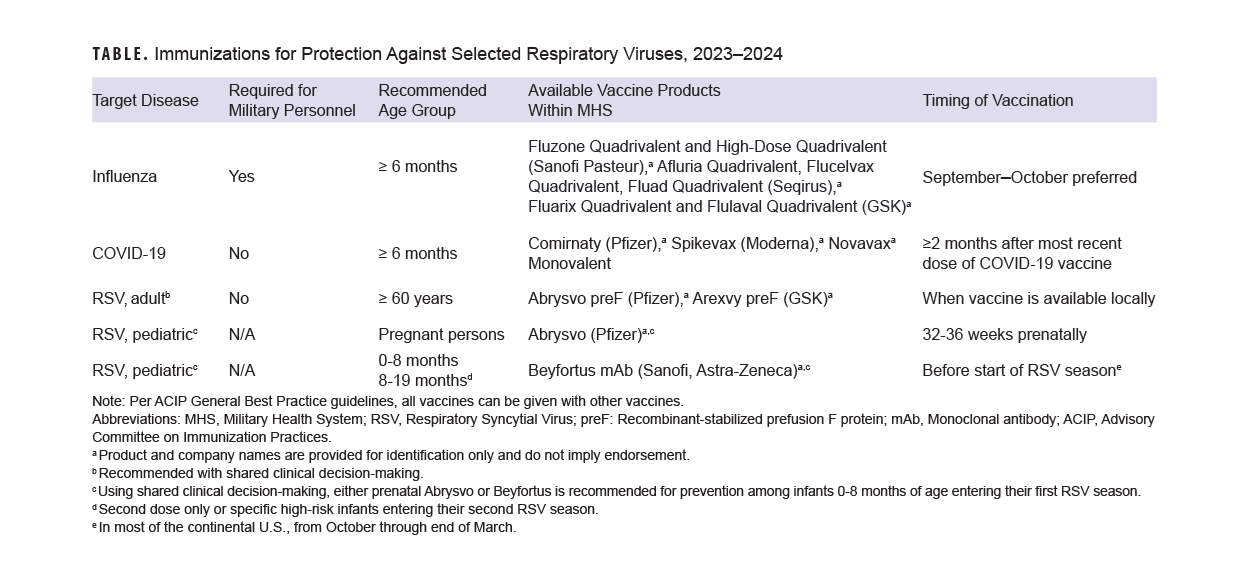
With children returned to school, another respiratory infection season approaches. This year, however, health care providers have new tools to prevent these infections in their patients, in the form of newly licensed or updated vaccines and immunotherapeutics against RSV and SARS-CoV-2. Revised recommendations for annual influenza vaccines will potentially also allow more people to easily receive these products.6
Yearly influenza vaccination is recommended for almost all persons aged 6 months or older (including pregnant persons)6 and is a requirement for both military personnel and all health care personnel working in military medical facilities.7 Data from the 2019-2020 influenza season show that influenza vaccination of U.S. Department of Defense beneficiaries reduced influenza disease by 40-60%, and by 12-31% among military members; for methodological reasons, military vaccine effectiveness may be underestimated.8
The U.S. Centers for Disease Control and Prevention states that the preferred time for vaccination for most persons who need only one dose of influenza vaccine is September or October. Vaccination should continue throughout the season, however, for as long as influenza viruses are circulating and vaccination is available. For children aged 6 months through 8 years, as well as pregnant persons in their third trimester, specific recommendations for timing of vaccination should be consulted.6
A notable change this year, which expands the population eligible for more ready vaccination, is the recommendation that all persons with egg allergy who are aged 6 months or older can receive influenza vaccine without special precautions. Regardless of the severity of prior egg reaction, egg allergy requires no additional safety measures for influenza vaccination. Observational data from numerous sources have documented the extreme rarity of anaphylactic-type allergic reactions to influenza vaccine in persons with egg allergy.6 Consistent with best practice recommendations, all vaccines including influenza should be administered in settings where rapid recognition and treatment of acute hypersensitivity reactions can occur.9
Vaccines against SARS-CoV-2, the virus which causes COVID-19 disease and its sequelae, are in a transitional period. Bivalent formulations (which should no longer be used) have been replaced by monovalent formulations based on the recent XBB.1.5 variant of the Omicron virus lineage.10 Both protein and mRNA-based vaccines are available this fall.
The Food and Drug Administration has approved monovalent vaccines for use as a single dose in all persons 5 years of age and older, provided that two months have elapsed following any prior COVID-19 vaccination. Infants and children 6 months through 4 years of age may receive 1, 2, or 3 doses of a monovalent vaccine, depending on COVID-19 vaccination history and which vaccine is used.10 The CDC is recommending the newly available monovalent COVID-19 vaccines for everyone 6 months and older.
In explaining its recommendations, the CDC noted that while older and immunocompromised individuals are at highest risk for hospitalization and death due to COVID-19, serious outcomes continue among healthy children and adults.11,12 Data indicate that, among those eligible for vaccination, individuals over age 75 and between 65-74 years of age have the highest hospitalization rates; continued vaccination of working-age adults (ages 18-49, the group that constitutes most military personnel) could prevent as many as 414 added hospitalizations over 6 months per 1 million doses administered.11
While it is well-documented that breakthrough infections can occur with COVID-19 vaccines, a recent evidence synthesis found that vaccination against Omicron variants was nearly 90% effective at preventing hospitalization due to COVID-19 within six weeks, with 71% effectiveness up to 16 weeks after vaccination.13 The CDC has estimated that for every 1 million COVID-19 doses administered to adolescents over a period of six months, up to 95 hospitalizations, 19 ICU admissions, and one death can be prevented.11 These estimates, complemented by a comprehensive benefit and risk assessment of COVID-19 vaccination provided by the CDC, constitute reliable evidence supporting the CDC Advisory Committee on Immunization Practices recommendation.14
RSV, long known as a widespread and potentially serious pediatric pathogen, is also a known cause of serious illness in older adults.15 The 2 new RSV vaccines, with recent FDA approval and CDC recommendation,16 are a new addition to the armamentarium against respiratory viruses and their complications. Both vaccines use inactivated RSV proteins to stimulate active immune response.
In clinical trials, both vaccines showed high efficacy in preventing symptomatic lower respiratory infection with RSV. Two-season interim analyses showed 81.0% efficacy of the Pfizer vaccine against medically-attended lower respiratory tract disease, and 77.5% efficacy of the GSK vaccine against the same outcome.16 The CDC ACIP recommends a single dose of either vaccine in persons aged 60 and over, using what it terms “shared clinical decision-making.”16 This phrase generally describes health care provider discussion of vaccination options with their patients, taking into account their preferences, as well as medical fragility, advanced age, and other clinical characteristics that may increase risk of severe outcomes from RSV.17 One of the newly-licensed RSV vaccines was also approved by the FDA for vaccination of pregnant persons for prevention of RSV infection in their infants, and the CDC has recently provided usage recommendations.18
Nirsevimab, a long-acting monoclonal antibody designed to prevent severe RSV lower respiratory tract disease in infants and children under 20 months of age, was recently licensed by the FDA. ACIP recommends nirsevimab for all infants under 8 months of age either born during or entering their first RSV season, in addition to infants and children aged 8 to 19 months at increased risk of severe RSV disease who are entering their second RSV season.19 Children at high risk include those with chronic lung disease requiring specific types of medical support, with severe immune compromise, or cystic fibrosis-meeting clinical criteria, in addition to children of American Indian or Alaskan Native heritage.
An inherent limitation of Nirsevimab is that as an antibody product rather than a true vaccine: It provides only passive immunity and does not result in longer-term immunologic memory. It is also not a treatment for RSV disease. Compared to palivizumab, a mAb previously approved by the FDA for RSV prevention in children up to 2 years of age,20 Nirsevimab has advantages of single dose administration, rather than requiring a 5-dose series, and is recommended for all infants entering RSV season instead of only certain high-risk pediatric patients. Maternal vaccination with the licensed and recommended RSV vaccine is an additional option for protection of all infants.18
While use of influenza, COVID-19, and RSV vaccine products are primary prevention measures against the “tripledemic,” the strategy has limitations. Only the influenza vaccine is currently required for military personnel. Only persons aged 60 years and older and children under 2 years of age can receive active and passive immunization against RSV, respectively. While preliminary laboratory evidence suggests that updated COVID-19 vaccines will offer cross-protection against recently-circulating SARS-CoV-2 Omicron virus variants such as BA.2.86,21 it is unknown how clinically effective the newly updated formulations will be.
While shared vaccine decision-making for RSV protection is a patient-centered approach, it is also more time-consuming and may result in lower overall vaccine uptake. For example, the shared decision-making model precludes standing orders for RSV vaccination of eligible adults within the MHS.
Because none of this group of vaccines is expected to produce sterilizing immunity (i.e., absolute protection against infection), additional voluntary personal protection methods for preventing respiratory virus transmission are warranted. Recent DOD Force Health Protection Guidance includes recommendations for frequent use of hand hygiene and covering the mouth and nose when coughing or sneezing.22 Health care providers should refrain from direct patient care while ill with respiratory symptoms, and may wish to consider wearing a securely-fitting mask for the duration of the winter respiratory virus season. Enhancing ventilation in indoor settings, where feasible, may also limit respiratory virus spread. Treatment facilities can monitor local respiratory virus circulation conditions that could affect testing and mitigation strategies by consulting DOD resources from Armed Forces Health Surveillance Division, Global Emerging Infections Surveillance, and CDC sites including Fluview and the Covid Data Tracker.
Evidence-based methods for enhancing immunization (including standing orders where applicable and offers of vaccination at all visits) should be implemented. Patients should be screened for eligibility for all three vaccines during fall and winter season visits (Table). Consistent with CDC best practices, co-administration of recommended vaccines at the same visit is recommended.9 Individuals who received the Southern Hemisphere influenza vaccine earlier this year should receive the Northern Hemisphere formulation this fall, as long as it has been 30 days since their most recent dose of influenza vaccine.
Author affiliations
Immunization Healthcare Division, Public Health Directorate, Defense Health Agency, Falls Church, VA: CAPT Iskander, LTC Martinez, Lt Col Hsu.
Acknowledgements
Cecilia Mikita, MD, MPH, and Margaret Ryan, MD, MPH, Immunization Healthcare Division, Public Health Directorate, Defense Health Agency, Falls Church, VA.
Disclaimer
The views expressed in this document are those of the author(s) and do not necessarily reflect the official opinion, policy, nor position of the Defense Health Agency, the Department of Defense, or the U.S. Government. The mention of any non-federal entity or its products is for informational purposes only, and is not to be construed, implied, or interpreted, in any manner, as federal endorsement of that non-federal entity or its products.
References
- Armed Forces Health Surveillance Division. Absolute and relative morbidity burdens attributable to various illnesses and injuries among active component members, U.S. Armed Forces, 2022. MSMR. 2023;30(6):3-11.
- Armed Forces Health Surveillance Division. Update: absolute and relative morbidity burdens attributable to various illnesses and injuries among non-service member beneficiaries of the Military Health System, 2022. MSMR. 2023;30(7):11-20.
- Patel TA, Jain B, Raifman J. Revamping public health systems: lessons learned from the “tripledemic.” Am J Prev Med. 2023:S0749-3797(23)00339-2.
- Bixler D, Miller AD, Mattison CP, et al. SARS-CoV-2-associated deaths among persons aged <21 years–United States, February 12-July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1324-1329.
- Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics. 2018;141(4):e20172918.
- Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices–United States, 2023-24 influenza season. MMWR Recomm Rep. 2023;72(RR-2):1-25.
- U.S. Department of Defense Immunization Program. U.S. Department of Defense Instruction 6205.02. Feb. 27, 2023.
- Sayers DR, Iskander JK. Influenza vaccine effectiveness and test-negative study design within the Department of Defense. Mil Med. 2023;usac436.
- Kroger A, Bahta L, Long S, Sanchez P. General Best Practice Guidelines for Immunization. Accessed Sep. 13, 2023. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf
- U.S. Food and Drug Administration. Press Announcements. Sep. 11, 2023. Accessed Sep. 13, 2023. https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating
- Centers for Disease Control and Prevention. Respiratory Viruses. Sep. 12, 2023. Accessed Sep. 13, 2023. https://www.cdc.gov/respiratory-viruses/whats-new/covid-vaccine-recommendations-9-12-2023.html
- Regan JJ, Moulia DL, Link-Gelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged ≥6 months: recommendations of the Advisory Committee on Immunization Practices–United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;ePub.
- Wu N, Joyal-Desmarais K, Ribeiro PAB, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. 2023;11(5):439-452.
- Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices. Sep. 12, 2023. Accessed Oct. 18, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/2+slides-2023-09-12/11-COVID-Wallace-508.pdf
- El Sherif M, Andrew MK, Ye L, et al. Leveraging influenza virus surveillance from 2012 to 2015 to characterize the burden of respiratory syncytial virus disease in Canadian adults ≥50 years of age hospitalized with acute respiratory illness. Open Forum Infect Dis. 2023;10(7):ofad315.
- Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices–United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:793-801.
- Havers FP, Whitaker M, Melgar M, et al. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus–RSV-NET, 12 states, July 2022-June 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1075-1082.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices–United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;ePub.
- Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices–United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:920-925.
- Caserta MT, O’Leary ST, Munoz FM, Ralston SL, Committee on Infectious Diseases. Palivizumab prophylaxis in infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2023;152(1):e2023061803.
- Centers for Disease Control and Prevention. Respiratory Viruses. Sep. 15, 2023. Accessed Sep. 18, 2023. https://www.cdc.gov/respiratory-viruses/whats-new/covid-19-variant-update-2023-09-15.html
- Memorandum for Senior Pentagon Leadership, Force Health Protection Guidance. Coronavirus Disease 2019 and Other Infectious Respiratory Diseases. Jul. 26, 2023.