The DHA Research and Engineering Directorate's Research Support Division supports and enables enterprise-wide scientific studies, research activities, and analyses to advance research priorities. The division performs research support functions in joint clinical investigations, research protections, and implementation science, and translates the latest research to inform clinical practice and policy.
RSD has gathered the following resources to help investigators navigate the DHA research process in the areas of surveys and questionnaires, human and animal research review, data sharing agreements, funding, general resources, database resources, and medical library resources. This list is not all inclusive as there may be other information needed for specific types of medical research such as animal and laboratory. To request updates or changes to this information, please contact the Clinical Investigations Program Office at dha.ncr.j-9.mbx.cip-office@health.milEmail CIPO.
R&E RSD Points of Contact
- Clinical Investigations Program OfficeOpens CIPO page: Provides funding and policy recommendations, and oversight and management for clinical investigations programs at military medical centers, hospitals, and clinics. Contact via email: dha.ncr.j-9.mbx.cip-office@health.milEmail CIPO
- Office of Research ProtectionsOpens ORP page: The Human Research Protections Program for DHA Headquarters that supports the DHA Institutional Official for human and animal use protocols and protection. Contact via email: dha.ncr.dha-cs-mgt.mbx.hrpp@health.milEmail ORP
- Implementation ScienceOpens IS page: Provides support and consultation on using implementation science methods to promote the adoption and integration of evidence-based practices into health care in the MHS, thereby improving health outcomes for service members and MHS beneficiaries. Contact via email: dha.ncr.j-9.mbx.isb@health.milEmail IS
- Military Health System ResearchMilitary Health System Research: Fosters health system research that improves health care delivery in the MHS by administering research grants that focus on factors that impact health outcomes, including cost, quality, variation, and policies. Contact via email: dha.ncr.j-9.mbx.hsr@health.milEmail MHSR
- National Museum of Health and MedicineOpens NMHM site: Collects, preserves, and presents the nation's medical and biomedical historical assets, emphasizing military collections-based research and interpretation. Contact via email: USArmy.Detrick.MEDCOM-USAMRMC.List.Medical-Museum@health.milEmail NMHM
R&E Centers of Excellence
Established to provide the Department of Defense with the ability to speed the advancement of scientific knowledge and evidence-based practices for diagnosis and treatment of diseases and conditions that impact military personnel and their families with the help of a “critical mass” of experts.
- Hearing Center of ExcellenceOpens HCE site: Fosters and promotes the prevention, diagnosis, mitigation, treatment, rehabilitation, and research of hearing loss and auditory injury.
- Vision Center of ExcellenceOpens VCE site: Leads and advocates for programs and initiatives with the following three inter-related goals: to improve vision health, optimize readiness, and enhance quality of life for service members and veterans.
- Traumatic Brain Injury Center of ExcellenceOpens TBICOE site: A congressionally mandated collaboration of the Departments of Defense and Veterans Affairs to promote state-of-the-science care from point-of-injury to reintegration for service members, veterans, and their families to prevent and mitigate consequences of mild to severe traumatic brain injury.
- Psychological Health Center of ExcellenceOpens PHCOE site: PHCoE collaborates across the Department of Defense and with the Department of Veterans Affairs and other agencies to provide leadership and expertise to inform policy and drive improvements in psychological health outcomes.
- Extremity Trauma and Amputation Center of ExcellenceOpens EACE site: The EACE leads the advancement of extremity trauma related discovery and clinical practice to optimize outcomes of service members, veterans, and beneficiaries while meeting the needs of the Combatant Commands and the Military Health System.
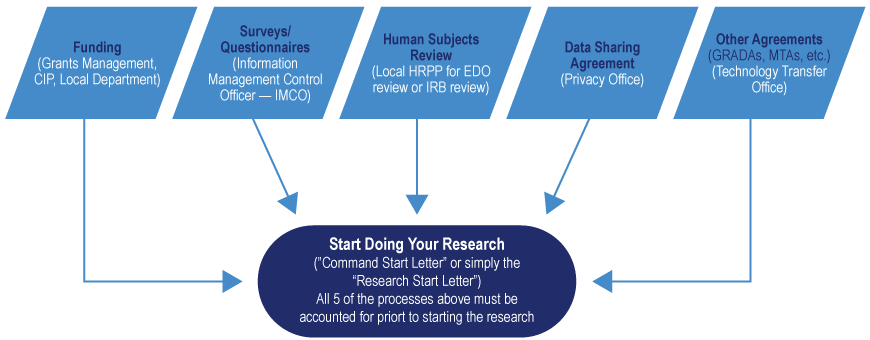
Surveys and Questionnaires
Surveys and questionnaires may need to be reviewed and approved for use by the Information Management Control Officer. Human subjects research protocols (such as drug, vaccine, or procedures research that uses a survey initially or in follow-up to assess subjects’ facts or opinions) are exempt from PRA review and approval by DoDM 8910.01 Vol 1goes to WHS and Vol 2goes to WHS as well as DoDI 3216.02goes to WHS.
Information Management Control Officer: Oversees the management, control, approval processing, and tracking of DOD Internal website and Public Information Collections. Surveys and questionnaires may need to be reviewed and approved for use by the IMCO in accordance with DoDM 8910.01 “DoD Information Collections Manual: Procedures for DoD Public Information Collections” Volume 1 and Volume 2Opens DOD directive.
Human and Animal Research Oversight Offices
- Office of Research ProtectionsOffice of Research Protections: The Human Research Protections Program for DHA Headquarters. They support and encourage research, including human subjects research. DoDI 3216.02 “Protection of Human Subjects and Adherence to Ethical Standards in DoD-Conducted and–Supported ResearchOpens DODI PDF.
- Department of the Air Force Research Oversight and Compliance Office: Oversees activities for both the US Air Force and US Space Force (i.e., collectively, the Department of the Air Force). Includes both the Animal Research Oversight and Compliance office and the DAF Component Office for Human Research Protections. They provide human research protection consultations and reviews for any DAF conducted and supported human research, use of animals in training, and use of animals in RDT&E.
- External Website: https://www.airforcemedicine.af.mil/Organizations/AF-Research-Oversight-Compliance/Opens DAF COHRP site
- Department of the Navy Human Research Protection ProgramOpens DON HRPP site: DON HRPP's mission is to ensure the ethical treatment of human subjects in DON-conducted or -supported research by promoting adherence to the ethical principles, laws, regulations, and policies that protect human subjects. DON HRPP’s vision is to preserve the rights and welfare of human subjects in the Navy and Marine Corps.
- Department of the Army Human Research Protections Office: AHRPO’s mission is to provide leadership in the protection of the rights and welfare of subjects involved in research conducted or supported by the Department of the Army. AHRPO develops Army-level policy, negotiates new and oversees existing DOD Assurances, provides clarification and guidance, develops educational programs and materials, maintains regulatory oversight, and provides advice on ethical and regulatory issues in biotechnological, social behavioral/academic, and engineering research.
- External Website: https://ahrpo.army.mil/Goes to AHRPO site
- Office of Human and Animal Research OversightGoes to MRDC website: OHARO ensures that U.S. Army Medical Research and Development Command conducted, contracted, sponsored, supported or managed research and U.S. Army Medical Command investigations involving human subjects, human anatomical substances or animals are conducted in accordance with federal, DOD, Army, USAMRDC, and international regulatory requirements.
- External Website: https://ahrpo.army.mil/Goes to AHRPO site
Human Subjects Review
Research protocols are required to be submitted through an electronic institutional review board for either an IRB review (non-exempt research) or an EDO review (exempt research or private investigator/quality improvement/evidence-based practice). The five DHA Institutional Review Boards include:
- Brooke Army Medical Center Institutional Review BoardOpens BAMC site: Located in San Antonio, Texas, BAMC IRB provides IRB services to Darnall Army Medical Center (TX), William Beaumont Army Medical Center (TX), Wilford Hall Ambulatory Surgical Center (TX), 81st Medical Group (MS), 55th Medical Group (NE), and Naval Hospital Pensacola (FL).
- Naval Medical Center Portsmouth IRB: Located in Portsmouth, Virginia, NMCP IRB provides IRB services to Martin Army Community Hospital (GA), Naval Hospital Jacksonville (FL), Blanchfield Army Community Hospital (KY), Eisenhower Army Medical Center (GA), Keller Army Community Hospital (NY), Womack Army Medical Center (NC), and Naval Medical Center Camp Lejeune (NC). Contact via email: usn.hampton-roads.navhospporsva.list.nmcp-irboffice@health.milEmail Naval Medical Center
- Naval Medical Center San Diego IRB: Located in San Diego, California, NMCSD IRB provides IRB services to Naval Hospital Twentynine Palms (CA), Naval Hospital Camp Pendleton (CA), Mike O’Callaghan Military Medical Center (NV), 96th Medical Group (FL), Evans Army Community Hospital (CO), and 375 Medical Group (IL). Contact the Human Protections Administrator, 34800 Bob Wilson Drive, San Diego, CA 92134, Telephone: 619-532-8125
- Madigan Army Medical Center IRBOpens MAMC site: Located in Tacoma, Washington, MAMC IRB provides IRB services to Naval Hospital Bremerton (WA), David Grant USAF Medical Center (CA), and Tripler Army Medical Center (HI).
- Walter Reed National Military Medical Center IRBOpens WRNMMC site: Located in Bethesda, Maryland, WRNMMC IRB provides IRB services to the Joint Pathology Center, 11th Medical Group (MD), Naval Health Clinic Annapolis (MD), Naval Health Clinic Quantico (VA), Fort Belvoir Community Hospital (VA), and Wright-Patterson Medical Center (OH).
Data Sharing Agreements
Health Insurance Portability and Accountability Act issues are handled through the Privacy Office, which is a two-step process. If you are going to be using DHA medical data that is collected from a patient, step 1 is to either obtain authorization from the patient to access their data, or obtain a waiver of HIPAA from a Privacy Board (most often this is an IRB). The Data Sharing agreement is step 2 of this process that follows the authorization or waiver of authorization determination.
Funding
In most military treatment facilities, funding for the actual execution of research comes from either the specific department that you see the patient in or through another funding source. Larger institutions may have Grant Managers with an office to assist you with getting funding. Below are some resources you can use to locate funding for your research.
- Clinical Investigations Program OfficeOpens CIPO page: Facilitates research and training to support Graduate Health Sciences Education and other allied health programs of the military services, to further enhance patient care in the MHS, and to contribute to medical readiness solutions.
- Grants.govOpens Grants.gov site: The mission of Grants.gov is to provide a common website for federal agencies to post discretionary funding opportunities and for grantees to find and apply to them. (Search “12.420” - keyword for military medical research)
- U.S. Army Medical Research and Development CommandU.S. Army Medical Research and Development Command: Manages and executes research in five basic areas: military infectious diseases, combat casualty care, military operational medicine, chemical biological defense, and clinical and rehabilitative medicine. MRDC is program coordinator for DOD medical research programs focused on the prevention, mitigation, and treatment of blast injuries as well as the manager for the Joint Trauma Analysis and Prevention of Injury in Combat program which informs solutions that prevent or mitigate injury during the full range of military operations, by collaborative collection, integration, analysis, and storage of data from operations, intelligence, materiel, and medical sources. MRDC funding opportunities include:
- U.S. Army Medical Research and Development Command Broad Agency AnnouncementOpens USAMRAA site: USAMRDC BAA is a competitive solicitation procedure intended to solicit extramural research and development ideas. Research funded through the USAMRDC BAA benefits and informs both military and civilian medical practice and knowledge. Research proposals and applications are sought from national, international, for-profit, non-profit, public, and private organizations. The USAMRDC BAA is a continuously open announcement; pre-proposals/pre-applications and proposals/applications may be submitted at any time throughout the 5-year period. (Must register at https://ebrap.org/ to apply).
- Congressionally Directed Medical Research ProgramsOpens CDMRP site: Fills research gaps by funding high impact, high risk, and high gain projects that other agencies may not venture to fund. CDMRP receives about $1.5 billion of congressionally directed medical research dollars for a large variety of medical problems each year. It is encouraged to check here first for research opportunities in specific areas of interest.
- Military Infectious Diseases Research ProgramOpens MIDRP site: Plans, coordinates, and oversees for the DOD requirements-driven medical solutions that prevent, predict, and treat infectious disease threats to the total force maximizing warfighter readiness and performance.Opens CCCRP site
- Combat Casualty Care Research ProgramOpens CCCRP site: Reduces mortality and morbidity resulting from injuries on the battlefield through the development of new life-saving strategies, new surgical techniques, biological and mechanical products, and the timely use of remote physiological monitoring.
- Military Operational Medicine Research ProgramOpens MOMRP site: Develops effective countermeasures against stressors to maximize health, performance, and fitness. MOMRP’s mission is to protect the soldier at home and on the battlefield.
- Medical Technology Enterprise Consortium: A public-private partnership that is managed by the Department of Defense and serves as an Other Transaction Authority to promote the development and delivery of innovative medical technologies to improve the health and safety of military personnel, veterans, and civilians. MTEC research is focused on technologies that can prevent injuries and accelerate the development of revolutionary medical solutions. Telephone: (843) 760-4083
- Military Health System ResearchMilitary Health System Research: Supports research projects with potential to innovate and improve the military health care system through annual grant awards. Each funding cycle MHSR publishes a Notice of Funding Opportunity on Grants.gov seeking rigorous intramural and extramural health systems research based on MHSR and clinical research priorities.
- National Institutes of HealthOpens NIH site: NIH is the largest public funder of biomedical research in the world. Information on grants and funding, such as due dates and how to apply, is provided.
- Small Business Innovation Research Program and Small Business Technology Transfer ProgramOpens SBIR site: Supports scientific excellence and technological innovation through the investment of federal research funds in critical American priorities to build a strong national economy.
General Resources
- Defense Acquisition University Contracting Officer’s Representative Community of PracticeOpens DAU site is for all members of the COR community, to include CORs, program managers, contracting officers, contract specialists, COR supervisors, and quality assurance personnel such as COR coordinators and quality assurance program coordinators. Resources for CORs, such as the latest DOD policies, DOD Instruction for COR standards, guides, tools, training, and events, are available. DAU COR hosts regular office hours on a weekly basis.
- Electronic Institutional Review Board: Centrally hosted enterprise-wide web-based application used to manage the lifecycle of research, review, and oversight process for DOD research, as well as unique DOD reviews.
- Military Health System Medical LibraryMilitary Health System Medical Library: Provides access to high-quality, evidence-based medical, nursing, and allied health information resources for MHS personnel.
- Privacy, Information Collection, and Human Research toolPrivacy, Information Collection and Human Research tool: Provides guidance on requirements related to collecting, using, and releasing information on individuals for research and related purposes. The tool may help you determine if your activity is research involving human subjects and will provide you with a broad overview of the compliance requirements.
- Working Information Systems to Determine Optimal Management training courseGoes to Working Information Systems to Determine Optimal Management training course: WISDOM provides guidance for MHS managers, data analysts and policy makers in the use of MHS data in support of operational questions, management decisions and corporate goals.
Database Resources
- Defense Manpower Data Center Reporting System:Opens DMDC site Reporting website that provides authorized users with the ability to view standard reports or to make custom data requests. The self-service data within this system is based on summarized personnel information from DMDC's data holdings. DMDCRS is designed for use by the U.S. federal government (military and civilian), U.S. federal government contractor, and various other support organization employees. Contact the DMDCRS Help Desk via email: dodhra.dodc-mb.dmdc.mbx.dmdcrs-helpdesk@mail.milEmail DMDCRS
- Defense Medical Epidemiology DatabaseDefense Medical Epidemiology Database: DMED provides remote access to a subset of data contained within the Defense Medical Surveillance System. DMSS contains up-to-date and historical data on diseases and medical events (e.g., hospitalizations, ambulatory visits, reportable diseases) and longitudinal data relevant to personnel characteristics and deployments experience for all active and reserve component service members. The DMED application provides a user-friendly interface to perform queries regarding disease and injury rates and relative burdens of disease in active component populations. Contact the DMED Administrator via email: dha.ncr.health-surv.mbx.afhs-ea-reports@health.milEmail DMED
- Defense & Veterans Eye Injury Vision RegistryOpens VCE site: DOD managed clinical health registry that provides the DOD and VA vision care community with DOD and VA ocular clinical and related data to track significant eye injuries of active-duty service members, support coordination of care, and support longitudinal studies and analysis on prevention, preservation, and restoration of the visual system. DVEIVR data and reports offer MHS researchers and medical personnel accurate information which improves eye care in support of a ready medical force.
- DOD Cancer Registry ProgramOpens DODCR fact sheet: Enterprise data application and repository supporting MHS cancer treatment and research.
- DOD Trauma RegistryOpens DODTR site: Web-based data collection tool that supports U.S. military performance improvement initiatives with global collection and aggregation of combat casualty care epidemiology, treatments, and outcomes. The trauma data registry captures and documents, in electronic format, information about the demographics, injury-producing incident, diagnosis and treatment, and outcome of injuries sustained by U.S./non-U.S. military and U.S./non-U.S. civilian personnel in wartime and peacetime from the point of injury to final disposition. Contact the Department of Defense Center of Excellence for Trauma, JTS Registry Management Branch via email: dha.jbsa.healthcare-ops.list.jts-trauma-log@health.milEmail DODTR
- Health Artifact and Image Management SolutionHealth Artifact and Image Management Solution: HAIMS provides the Departments of Defense and Veterans Affairs health care providers global visibility and access to artifacts and images generated during the health care delivery process.
- Joint Hearing Loss & Auditory System Injury RegistryOpens HCE site: Combines clinical episodes of care from both DOD and VA audiograms, demographic, deployment, theatre trauma, and non-trauma data. All information is in computable fields to promote analysis, research, performance improvement, and continuity of care. Contact the HCE Information Management Branch via email: dha.ncr.j-9.list.hce-im@health.milEmail HCE IM
- Military Health Systems Information Platform: Provides clinical information data warehousing modules that enable DHA to monitor, extract, and make available clinical/business data from MTFs. MIP servers contain and process military medial data for use in data analysis and patient care.
- Soldier Performance, Health, and Readiness DatabaseOpens SPHERE site: A high-resolution epidemiologic research tool that serves as a significant resource for identifying risk/protective factors and adverse health outcomes and for evaluating intervention strategies in Army personnel. The SPHERE is a vast data repository that combines U.S. Army population data from multiple disparate Department of Defense agencies and is housed and managed within the USAMRDC's U.S. Army Research Institute of Environmental Medicine’s Military Performance Division by a team of epidemiologists, analysts, and database managers. Contact SPHERE via email: usarmy.natick.medcom-usariem.mbx.usariem-sphere@health.milEmail SPHERE
Medical Library Resources
- Air Force Medical Service Virtual LibraryOpens AFMS site: The AFMS Virtual Library provides resources to assist with literature searches, interlibrary loan requests, assistance with accessing library resources, and offers comprehensive database access. For questions regarding Virtual Library navigation, interlibrary loans, literature searches, and database issues, please visit the “Contact Us” section on the Virtual Library home page.
- MHS Medical LibraryMHS Medical Library: The MML provides access to high-quality, evidence-based medical, nursing, and allied health information resources for MHS personnel. The MML ensures that all personnel have access to core, essential resources regardless of duty station. Visit DynaMedEx under “Popular Databases” to find answers to clinical questions and earn continuing medical education and maintenance of certification credits by viewing topics. For questions, please visit the “Contact MML” section on the home page. A list of facility libraries and contact information can be found under “Other Medical Libraries” then “Facility Libraries.”
- Navy Medicine Electronic Library AccessNavy Medicine Electronic Library Access: NMeL offers access to UpToDate, PubMed, eJournals, eBooks, DynaMedex and Google Scholar.
- NOTE: If this is your first time accessing the NMeL, you must register for an account using your CAC-enabled computer and government email. You will then receive an email with a link to allow you to create a password for use with the system.
- For questions regarding registration, please visit the “Contact details for library services” section on the Registration page.
** Remember, it is a researcher’s responsibility to account for all processes and approvals in conducting medical research in the military! **